Propagation of Haemaria Discolor Via in Vitro Seed Germination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dating the Origin of the Orchidaceae from a Fossil Orchid with Its Pollinator
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6111228 Dating the origin of the Orchidaceae from a fossil orchid with its pollinator Article in Nature · September 2007 DOI: 10.1038/nature06039 · Source: PubMed CITATIONS READS 211 770 5 authors, including: Santiago R Ramírez Barbara Gravendeel University of California, Davis Leiden University, Naturalis Biodiversity Center & University of Applied Sciences L… 50 PUBLICATIONS 999 CITATIONS 208 PUBLICATIONS 2,081 CITATIONS SEE PROFILE SEE PROFILE Rodrigo B. Singer Naomi E Pierce Universidade Federal do Rio Grande do Sul Harvard University 109 PUBLICATIONS 1,381 CITATIONS 555 PUBLICATIONS 6,496 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Insect endosymbiont diversity View project Support threatened research Institutions from Southern Brazil (Rio Grande do Sul) View project All content following this page was uploaded by Barbara Gravendeel on 31 May 2014. The user has requested enhancement of the downloaded file. Vol 448 | 30 August 2007 | doi:10.1038/nature06039 LETTERS Dating the origin of the Orchidaceae from a fossil orchid with its pollinator Santiago R. Ramı´rez1, Barbara Gravendeel2, Rodrigo B. Singer3, Charles R. Marshall1,4 & Naomi E. Pierce1 Since the time of Darwin1, evolutionary biologists have been fas- subfamily showed that the size, shape and ornamentation of the cinated by the spectacular adaptations to insect pollination exhib- fossil closely resemble those of modern members of the subtribe ited by orchids. However, despite being the most diverse plant Goodyerinae, particularly the genera Kreodanthus and Microchilus family on Earth2, the Orchidaceae lack a definitive fossil record (Supplementary Table 1). -

Effects of Different Factors on Adventitious Bud Induction from Stem Explants of Ludisia Discolor
E3S Web of Conferences 245, 03024 (2021) https://doi.org/10.1051/e3sconf/202124503024 AEECS 2021 Effects of different factors on adventitious bud induction from stem explants of Ludisia discolor Ying Liu1, Xiaohao Li1, Jingye Chen1, Yingbin Xue2* and Yinling Zhu2* 1College of Coastal Agricultural Sciences, Guangdong Ocean University, Zhanjiang 524088, P.R. China 2College of Chemistry and Environment, Guangdong Ocean University, Zhanjiang 524088, P.R. China Abstract. In this study, the stem explants of Ludisia discolor were used as experimental materials to investigate the effects of 6-BA, NAA, Cu2+ and Ag+ on the induction of adventitious bud regeneration, and to analyze the most suitable culture conditions for stem explants regeneration. The results showed that when the medium was supplemented with 1.0 mg/L 6-BA, 0.75 mg/L NAA, 0.25 mg/L CuSO4, or 6.4 mg/L AgCl, the best regeneration effects would be gained, respectively. And the adventitious bud regeneration rate reached the maximum, which were 61.67%, 83.67%, 80.95% and 87.63%, respectively. The results of this study provided a theoretical basis for tissue culture and rapid propagation of L. discolor. 1 Introduction Ludisia discolor ((Ker-Gawl.) A. Rich.) is a medicinal 2 Materials and Methods plant belonging to Orchidaceae and the genus of Ludisia. It is often called "Goldenline Orchidaceae" [1]. Ludisia 2.1 Experimental materials discolor is mainly distributed in China, Vietnam, Malaysia, Thailand and other places [2]. It can be found The aseptic seedlings of L. discolor used in this study in northern and central Guangdong of China, Hong Kong, was provided by Professor Yang Yuesheng, College of Hainan, Guangxi, and southeast of Yunnan, and mainly Life Sciences, South China Agricultural University. -

Greater North Texas Orchid Society Dec
GREATER NORTH TEXAS ORCHID SOCIETY DEC Next Meeting: December 6 1 Mike brought the meeting to order at 3:10 with 25 people in attendance, 3 visitors. He announced the meeting next month is our semi-annual auction and dinner. Hi everyone, Please bring a dinner item to share with OfficeRS Presidents The year is almost over the group and plant(s) or orchid related and it has flown by. items to donate to the auction. Message It is time for our Rhonda introduced our speaker, second auction of the Charles Hess, whose topic of the day was PRESIDENT year. As most of you know the two auctions are “The Future of Conservation”. Major air Mike Beber our major fund raisers for our society. They help to pollution from burning forests; the bogs VICE PRESIDENT pay for speakers and other society related expenses. catch fire and are hard to put out emit- Rhonda Whitson Please bring orchids, potting medium, pots, or ting methane and carbon. Emissions are so large that on several days it has sur- SECRETARY anything else orchid related, to be placed in the Barbara McNamee auction. passed the daily emissions of the entire US Oh, and I haven’t mentioned the food! We will economy, has killed 10 people so far. For TREASURER be starting at 2pm this time and will have enough more information and/or how to help, see Kathy Halverson time to sit down, socialize, and enjoy real food, so this website: change.org - go online and SWROGA DIRECTORS bring anything you enjoy - soups, BBQ, sandwich- look see if anything interests you and sign Brandenburgs es, or surprise us! Most of you know I love to eat up! Charles art website is: NEWSLETTER EDITOR as much as I love orchids so I am looking forward orchidartbycharleshess. -

Orchiflora January 2015
Volume 5, Issue 4 Orchiflora January 2015 Announcements: Monthly General Meetings: 4th Wednes- day of each month (except July, August Speaker Series: 8:30-9:30 Floral Hall, VanDusen Gardens & December) at Van Dusen Floral Hall January 28, 2015: Terry Groszeibl “Paphiopedilums and their culture” Forestview Doors Open 6:30pm, Meeting starts at Gardens. IMPORTANT: Terry will take pre-orders until January 27, visit websitte at http://www.fvgardens.com/ and email your order to [email protected] or 7:30pm [email protected] February 25: AOS judging explained: 5 AOS judges will do their evaluations before the audi- ence; This outreach judging should help to demystify how orchid plants get awards. Bring Inside this issue: your orchids to the monthly meeting as usual. March 25: Calvin Wong will present on The 2015 Tokyo Dome Show. Calvin will share the stunning displays he encountered in February. Message From the President Pg 2 April 22: Alexey Tretyakov—Key Factors for Successful Growing on a Windowsill, Under Lights or in a Greenhouse May 27: Patricia Harding, Topic to be announced Christmas Party—26 November 2014 Pg 4 June 24: Roy Tokunaga from H&R (Hawaii), Topic to be announced Culture Class, held in the Cedar room, VanDusen Gardens from 6:30 to 8: 30 pm, all orchid How Santa Helped Illuminate Me— Pg 9 related questions welcome - culture class is a members’ only benefit February 9, 2015: Specialty Displays of your orchids, led by Daniel Kwok. Mr. Kwok has just Margaret Prat won an American Orchid Society Award for his display on a coral stone piece. -

Jewels of the Orchidaceae W
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Richmond University of Richmond UR Scholarship Repository Biology Faculty Publications Biology Spring 2016 Jewels of the Orchidaceae W. John Hayden University of Richmond, [email protected] Follow this and additional works at: http://scholarship.richmond.edu/biology-faculty-publications Part of the Biology Commons, and the Botany Commons Recommended Citation Hayden, W. John. "Jewels of the Orchidaceae ." Sempervirens Quarterly, Fall 2016, 6-7. This Article is brought to you for free and open access by the Biology at UR Scholarship Repository. It has been accepted for inclusion in Biology Faculty Publications by an authorized administrator of UR Scholarship Repository. For more information, please contact [email protected]. 6 Sempervirens , Fall 2016 By W. John Hayden, Botany Chair o temperate-zone plant enthusiasts, the orchid family seems more than a little strange. On the one hand, native orchids grow wild without assistance from people, they are rooted in the soil, and they survive freezing cold winter temperatures. On the other hand, the tropical or- chids that we encounter are ornamental plants, pampered by their human caregivers, cultured indoors in pots fi lled with fi r bark or other media designed to mimic the plants’ natural epiphytic habit, and, as a group, these ornamental tropical orchids have essentially zero tolerance to frost. Of course, their fl owers, fruits, and seeds defi ne them all as members of the orchid family, Orchidaceae, but from the perspective of how they actually live, and how we interact with them, native orchids and their tropical ornamental relatives seem utterly, profoundly, different. -
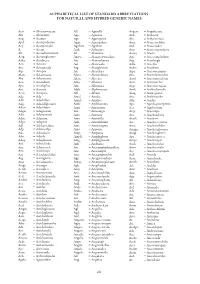
Orchid Name Abbreviations List
ALPHABETICAL LIST OF STANDARD ABBREVIATIONS FOR NATURAL AND HYBRID GENERIC NAMES Acw. = Aberconwayara All. = Aganella Angcst. = Angulocaste Abr. = Aberrantia Agn. = Aganisia Ank. = Anikaara Acp. = Acampe Agt. = Aganopeste Akr. = Ankersmitara Apd. = Acampodorum Agsp. = Agasepalum Anct. = Anoectochilus Acy. = Acampostylis Agubata = Agubata Atd. = Anoectodes A. = Aceras Aitk. = Aitkenara Ano. = Anoectogoodyera Ah. = Acerasherminium Al. = Alamania Anota = Anota Actg. = Aceratoglossum Agwa. = Alangreatwoodara Ayp. = Ansecymphyllum Acba. = Acinbreea Atc. = Alantuckerara Asg. = Anselangis Acn. = Acineta Aat. = Alaticaulia Aslla. = Ansellia Ain. = Acinopetala Atg. = Alatiglossum Asdm. = Ansidium Aip. = Aciopea Alc. = Alcockara Arpt. = Anteriocamptis Akm. = Ackermania Alxra. = Alexanderara Ahc. = Anterioherorchis Aks. = Ackersteinia Alcra. = Aliceara Atml. = Anteriomeulenia Aco. = Acoridium Alna. = Allenara Antr. = Anteriorchis Apa. = Acrolophia Aln. = Allioniara Atsp. = Anterioserapias Aro. = Acronia Alph. = Alphonsoara Anth. = Anthechostylis Acro. = Acropera Alv. = Alvisia Antg. = Antheglottis Ada = Ada Amal. = Amalia Anr. = Antheranthe Adh. = Adachilum Amals. = Amalias Alla. = Antilla Adg. = Adacidiglossum Amb. = Amblostoma Apr. = Apoda-prorepentia Adcm. = Adacidium Amn. = Amenopsis Aea. = Appletonara Adgm. = Adaglossum Am. = Amesangis Arcp. = Aracampe Adn. = Adamantinia Ams. = Amesara Ara. = Arachnadenia Adm. = Adamara Ame. = Amesiella Arach. = Arachnis Adps. = Adapasia Aml. = Amesilabium Act. = Arachnocentron Adl. = Adelopetalum Ami. = Amitostigma -

Toskar Newsletter
TOSKAR NEWSLETTER A Quarterly Newsletter of the Orchid Society of Karnataka (TOSKAR) Vol. No. 4; Issue: iv; 2017 THE ORCHID SOCIETY OF KARNATAKA www.toskar.org ● [email protected] From the Editor’s Desk TOSKAR NEWSLETTER 21st December 2017 Greetings for a merry Christmas and a Happy EDITORIAL BOARD (Vide Circular No. TOSKAR/2016 New year to all my friends! th Dated 20 May 2016) Well the show has come to an end and so also with that the year of 2017. I am told that it was yet another fantastc orchid show for the orchid lovers from Bengaluru and also the visitors. I really missed it, as Chairman I was away and requested Dr. Hegde to brief all the people who missed Dr. Sadananda Hegde with a note and pictures. Thanks Dr. Hegde. Organizing a show is a humongous task, it is not only logistcs, there are umpteenth issues to Members be coordinated and put together on tme. Well, the team has done it Mr. S. G. Ramakumar again, once again congratulatons for the entre team to have done it Mr. Sriram Kumar successfully for the fih tme. TOSKAR has successfully created interest amongst the hobbyists and which is also one of its objectves. Editor Winter has really set in here, for the last fortnight (from Dec 10 Dr. K. S. Shashidhar onwards tll now) the night temperatures are hovering between 12-14 C. In a way this is good for species from North East as thy need cool and Associate Editor dry nights, hopefully Bangalore growers should be happy with this. -

Surface Sterilization and Micropropagation of Ludisia Discolor
Biocatalysis and Agricultural Biotechnology 22 (2019) 101380 Contents lists available at ScienceDirect Biocatalysis and Agricultural Biotechnology journal homepage: http://www.elsevier.com/locate/bab Surface sterilization and micropropagation of Ludisia discolor Ranjetta Poobathy a,c, Rahmad Zakaria a, Vikneswaran Murugaiyah b, Sreeramanan Subramaniam a,d,* a School of Biological Sciences, Universiti Sains Malaysia, 11800, Gelugor, Pulau Pinang, Malaysia b School of Pharmaceutical Sciences, Universiti Sains Malaysia, 11800, Gelugor, Pulau Pinang, Malaysia c School of Biological Sciences, Quest International University Perak, 30250, Ipoh, Perak, Malaysia d Centre for Chemical Biology, Universiti Sains Malaysia, Bayan Lepas, Pulau Pinang, Malaysia ARTICLE INFO ABSTRACT Keywords: The jewel orchid Ludisia discolor, found in many parts of Asia, is valued due to its appearance and medicinal Micropropagation benefits. Existing surface sterilization protocols were found to be not successful in initiating in vitro cultures of Jewel orchid L. discolor in this study. Hence, a surface sterilization protocol, incorporating the use of 0.4% (w/v) mercury Ludisia discolor chloride, was developed to address this issue. This method yielded the lowest contamination (7.7%) and best Surface sterilization growth at 22.5%. Nodal segments of L. discolor produced the best growth when placed in half-strength semi-solid Acclimatization MS medium with 0.2% (w/v) activated charcoal, 8% (w/v) Mas banana cultivar homogenate, 3% (w/v) sucrose, À À À 3.5 g L 1 Gelrite, 1.0 mg L 1 1-naphthaleneacetic acid, and 0.1 mg L 1 thidiazuron, a confirmation of the pro tocol promoted by Shiau et al. (2005). All in vitro cultures of L. -

Little Known Houseplants That Anyone Can Grow
Little Known Houseplants That Anyone Can Grow By Mary Francis, Fairfax Master Gardener Intern There are many fine plants that are surprisingly easy to grow. Those featured in this article may not be suitable for houseplant beginners, yet they are most satisfying for those with some experience. These plants have key essential needs that, once met, vastly reward the grower. They are worth seeking out. Jewel Orchids Everyone is familiar with orchids, but have you ever encountered the jewel orchids? They are prized for the beauty of their leaves as the flowers are small and not author by showy. There are five genera of jewel orchids, all terrestrial, meaning they grow on the forest floor. Special photo: potting mix is available and often includes a mixture of Jewel Orchid Anoectochilis potting soil, sand, peat moss, sphagnum, perlite and cocoa fibers. They prefer a humid environment, so misting or even placing them in a terrarium is a good idea. The easiest to grow and the most commonly available jewel orchid is Ludisia discolor. Notable cultivars include ‘Red Velvet’ with red veins, and ‘Green Velvet’ which has bright green leaves with white veins. It can handle low light but prefers low to medium light for more showy leaves. It also tolerates overwatering or underwatering, thrives with orchid fertilizer and can even live in African violet potting soil. Flowers should be removed if they appear because the flowers weaken the plant. Jewel orchids of the genus Anoectochilus are also worthy of consideration. These plants have stricter requirements than Ludisia discolor yet are surprisingly easy to grow. -

Kakadu National Park Approved Plant List
KAKADU NATIONAL PARK - APPROVED PLANT LIST Kakadu National Park is a ‘Commonwealth reserve’ under the Environment Protection and Biodiversity Conservation Act 1999 (Cth) and is inscribed on the World Heritage List for outstanding cultural and natural values. As part of Kakadu National Park, the use and development of the Town of Jabiru must not be detrimental to the values of the park. To protect Kakadu’s unique environment, Regulations 12.20 and 12.21 of the Environment Protection and Biodiversity Conservation Regulations 2000 require that plants are not brought into Jabiru and cultivated or propagated unless they are included on the Approved Plant List. Approved plant species include a plant species that is: a) A native species of Kakadu National Park; or b) Specified on the Approved Plant List maintained by the Director of National Parks. All plants brought into Jabiru for domestic or commercial area gardens should be an approved plant species. The preferred option is to use species that are native to Kakadu. For information on these species please refer to the book Native Plants for Top End Gardens (2007 Smith). The approved plant species that are non-indigenous to Kakadu are those that are understood to not have the potential to spread into the park. The approved non-indigenous species may not necessarily be well-suited to the growing conditions in Jabiru. By using only approved plant species you will help to avoid potential biosecurity threats to the environmental values of Kakadu National Park. Please be aware that any gardening material brought into Jabiru provides an entry pathway for exotic ants, myrtle rust and other plant viruses and pathogens into the park. -
Orchid Research Newsletter 75 (PDF)
Orchid Research Newsletter No. 75 January 2020 Editorial Orchids are perhaps not the first thing that comes to mind when we think about climate change. Record temperatures, catastrophic droughts, melting glaciers, out-of- control bush fires, burning rainforests and other calamities are of more immediate concern. But when we focus on orchid conservation, it is obvious that climate change looms large. It seems likely that orchids are more vulnerable to climate change than most other plant groups, for the following reasons: (1). Since about 70% of all orchids are epiphytes, they are probably more likely to be affected by drought. Even if mature plants would be able to survive unusually severe droughts, one can imagine that seedlings would be much more vulnerable. If such droughts become too frequent, seedling recruitment will be compromised, and the orchids will die out. (2). Since all orchids go through a mycoheterotrophic stage, at least as as seedlings, they depend on the presence of the right fungi for their long-term survival. It could be that climate change affects these fungi in such a way that they are no longer available to particular orchid species. These will then gradually disappear from their habitats. (3). Similarly, since many orchids depend on highly specific pollinators, the effect of climate change on the availability of these pollinators may be significant. A chain is only as strong as its weakest link, and we do not know if it is the orchid, the fungus or the pollinator that is the weakest link. (4). Orchids tend to occur in sparse, widely dispersed populations. -
Orchidaceae) from Indonesia, and Fungal Association of Goodyera Section Goodyera
Phylogenetic Analyses of subtribe Goodyerinae and Revision of Goodyera section Goodyera (Orchidaceae) from Indonesia, and Fungal Association of Goodyera section Goodyera Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Lina Susanti Juswara, MSc. Graduate Program in Evolution, Ecology, and Organismal Biology The Ohio State University 2010 Dissertation Committee: Paul Fuerst, Advisor Laura Kubatko Hans Klompen Copyright by Lina Susanti Juswara 2010 Abstract Phylogenetic analyses using morphological and molecular data of the orchid subtribe Goodyerinae were performed using parsimony, maximum likelihood, and Bayesian methods. Two hypotheses, proposed by Dressler (1993) and Szlachetko (1995), were tested. The results showed that neither hypothesis can be supported, and that there are no morphological characteristics that can define groups within the subtribe Goodyerinae. Monophyletic groups within the subtribe cannot be defined using molecular information from three genes. Systematic revision of Goodyera section Goodyera from Indonesia was investigated using phenetic analyses (cluster analysis, non-metric multidimensional scaling), and discriminant function analysis. The results showed that, of eight forms initially recognized, only three independent taxa can be recognized by these analyses. The independent taxa are Goodyera bifida, G. procera, and G. reticulata. Six subspecies are recognized under Goodyera reticulata. They are G. reticulata subsp. colorata, G. reticulata subsp. gibbsiae, G. reticulata subsp. gemmata, G. reticulate subsp. pusilla, and G. reticulata subsp. reticulata. ii Fungal associations with members of Goodyera section Goodyera from Indonesia were examined using data from the fungal nuclear ITS region. Indonesian species of Goodyera section Goodyera have a broader range of fungal associates than do other taxa from the subtribe that had been previously studied.