Orchid Research Newsletter 75 (PDF)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Checklist of the Orchids of the Crimea (Orchidaceae)
J. Eur. Orch. 46 (2): 407 - 436. 2014. Alexander V. Fateryga and Karel C.A.J. Kreutz Checklist of the orchids of the Crimea (Orchidaceae) Keywords Orchidaceae, checklist of species, new nomenclature combinations, hybrids, flora of the Crimea. Summary Fateryga, A.V. & C.A.J. Kreutz (2014): Checklist of the orchids of the Crimea (Orchidaceae).- J. Eur. Orch. 46 (2): 407-436. A new nomenclature checklist of the Crimean orchids with 49 taxa and 16 hybrids is proposed. Six new taxa are added and ten taxa are excluded from the latest checklist of the Crimean vascular flora published by YENA (2012). In addition, five nomenclature changes are proposed: Epipactis persica (Soó) Nannf. subsp. taurica (Fateryga & Kreutz) Fateryga & Kreutz comb. et stat. nov., Orchis mascula (L.) L. var. wanjkovii (E. Wulff) Fateryga & Kreutz stat. nov., Anacamptis ×simorrensis (E.G. Camus) H. Kretzschmar, Eccarius & H. Dietr. nothosubsp. ticinensis (Gsell) Fateryga & Kreutz stat. nov., ×Dactylocamptis uechtritziana (Hausskn.) B. Bock ex M. Peregrym & Kuzemko nothosubsp. magyarii (Soó) Fateryga & Kreutz comb. et stat. nov., and Orchis ×beyrichii Kern. nothosubsp. mackaensis (Kreutz) Fateryga & Kreutz comb. et stat. nov. Moreover, a new variety, Limodorum abortivum (L.) Sw. var. viridis Fateryga & Kreutz var. nov. is described. Zusammenfassung Fateryga, A.V. & C.A.J. Kreutz (2014): Eine Übersicht der Orchideen der Krim (Orchidaceae).- J. Eur. Orch. 46 (2): 407-436. Eine neue nomenklatorische Liste der Orchideen der Krim mit 49 Taxa und 16 Hybriden wird vorgestellt. Sechs Arten sind neu für die Krim. Zehn Taxa, die noch bei YENA (2012) in seiner Checklist aufgelistet wurden, kommen auf der Krim nicht vor und wurden gestrichen. -

Evolution of Anatomical Characters in Acianthera Section Pleurobotryae (Orchidaceae: Pleurothallidinae)
RESEARCH ARTICLE Evolution of anatomical characters in Acianthera section Pleurobotryae (Orchidaceae: Pleurothallidinae) Audia Brito Rodrigues de AlmeidaID*, Eric de Camargo SmidtID, Erika Amano Programa de PoÂs-GraduacËão em BotaÃnica, Setor de Ciências BioloÂgicas, Universidade Federal do ParanaÂ, Curitiba, PR, Brazil * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Acianthera section Pleurobotryae is one of ten sections of the genus Acianthera and include four species endemic to the Atlantic Forest. The objective of this study was to describe com- paratively the anatomy of vegetative organs and floral micromorphology of all species of Acianthera section Pleurobotryae in order to identify diagnostic characters between them OPEN ACCESS and synapomorphies for the section in relation of other sections of the genus. We analyzed Citation: Almeida ABRd, Smidt EdC, Amano E roots, ramicauls, leaves and flowers of 15 species, covering eight of the nine sections of (2019) Evolution of anatomical characters in Acianthera section Pleurobotryae (Orchidaceae: Acianthera, using light microscopy and scanning electron microscopy. Acianthera section Pleurothallidinae). PLoS ONE 14(3): e0212677. Pleurobotryae is a monophyletic group and the cladistic analyses of anatomical and flower https://doi.org/10.1371/journal.pone.0212677 micromorphology data, combined with molecular data, support internal relationship hypoth- Editor: Suzannah Rutherford, Fred Hutchinson eses among the representatives of this section. The synapomorphies identified for A. sect. Cancer Research Center, UNITED STATES Pleurobotryae are based on leaf anatomy: unifacial leaves, round or elliptical in cross-sec- Received: August 24, 2018 tion, round leaves with vascular bundles organized in concentric circles, and mesophyll with Accepted: February 7, 2019 28 to 30 cell layers. -

Phylogenetic Placement of the Enigmatic Orchid Genera Thaia and Tangtsinia: Evidence from Molecular and Morphological Characters
TAXON 61 (1) • February 2012: 45–54 Xiang & al. • Phylogenetic placement of Thaia and Tangtsinia Phylogenetic placement of the enigmatic orchid genera Thaia and Tangtsinia: Evidence from molecular and morphological characters Xiao-Guo Xiang,1 De-Zhu Li,2 Wei-Tao Jin,1 Hai-Lang Zhou,1 Jian-Wu Li3 & Xiao-Hua Jin1 1 Herbarium & State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, P.R. China 2 Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650204, P.R. China 3 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun Township, Mengla County, Yunnan province 666303, P.R. China Author for correspondence: Xiao-Hua Jin, [email protected] Abstract The phylogenetic position of two enigmatic Asian orchid genera, Thaia and Tangtsinia, were inferred from molecular data and morphological evidence. An analysis of combined plastid data (rbcL + matK + psaB) using Bayesian and parsimony methods revealed that Thaia is a sister group to the higher epidendroids, and tribe Neottieae is polyphyletic unless Thaia is removed. Morphological evidence, such as plicate leaves and corms, the structure of the gynostemium and the micromorphol- ogy of pollinia, also indicates that Thaia should be excluded from Neottieae. Thaieae, a new tribe, is therefore tentatively established. Using Bayesian and parsimony methods, analyses of combined plastid and nuclear datasets (rbcL, matK, psaB, trnL-F, ITS, Xdh) confirmed that the monotypic genus Tangtsinia was nested within and is synonymous with the genus Cepha- lanthera, in which an apical stigma has evolved independently at least twice. -

Generic and Subtribal Relationships in Neotropical Cymbidieae (Orchidaceae) Based on Matk/Ycf1 Plastid Data
LANKESTERIANA 13(3): 375—392. 2014. I N V I T E D P A P E R* GENERIC AND SUBTRIBAL RELATIONSHIPS IN NEOTROPICAL CYMBIDIEAE (ORCHIDACEAE) BASED ON MATK/YCF1 PLASTID DATA W. MARK WHITTEN1,2, KURT M. NEUBIG1 & N. H. WILLIAMS1 1Florida Museum of Natural History, University of Florida Gainesville, FL 32611-7800 USA 2Corresponding author: [email protected] ABSTRACT. Relationships among all subtribes of Neotropical Cymbidieae (Orchidaceae) were estimated using combined matK/ycf1 plastid sequence data for 289 taxa. The matrix was analyzed using RAxML. Bootstrap (BS) analyses yield 100% BS support for all subtribes except Stanhopeinae (87%). Generic relationships within subtribes are highly resolved and are generally congruent with those presented in previous studies and as summarized in Genera Orchidacearum. Relationships among subtribes are largely unresolved. The Szlachetko generic classification of Maxillariinae is not supported. A new combination is made for Maxillaria cacaoensis J.T.Atwood in Camaridium. KEY WORDS: Orchidaceae, Cymbidieae, Maxillariinae, matK, ycf1, phylogenetics, Camaridium, Maxillaria cacaoensis, Vargasiella Cymbidieae include many of the showiest align nrITS sequences across the entire tribe was Neotropical epiphytic orchids and an unparalleled unrealistic due to high levels of sequence divergence, diversity in floral rewards and pollination systems. and instead to concentrate our efforts on assembling Many researchers have posed questions such as a larger plastid data set based on two regions (matK “How many times and when has male euglossine and ycf1) that are among the most variable plastid bee pollination evolved?”(Ramírez et al. 2011), or exon regions and can be aligned with minimal “How many times have oil-reward flowers evolved?” ambiguity across broad taxonomic spans. -

Atlanta Orchid Society Newsletter
The Atlanta Affiliated with the American Orchid Orchid Society, the Orchid Digest Corporation and the Mid-America Orchid Congress. Society 2001 Recipient of the American Orchid Society’s Distinguished Affiliated Bulletin Societies Service Award Newsletter Editor: Danny Lentz Volume 47: Number 3 www.atlantaorchidsociety.org March 2006 MARCH EVENTS The Meeting: 8:00 Monday, March 13 at Atlanta Botanical Garden David Mellard – Fertilizer and Water Quality (part 2) Please bring your handouts from the January meeting as you will need them for the remainder of David Mellard's talk about water quality and fertilizers. The second portion of the talk will cover in more detail the effect of Atlanta's low alkalinity water on growing conditions for orchids, particularly as it affects choosing the right fertilizer and understanding the importance of pH in the orchid mix. David will report on specific studies that have been done on orchid nutrition, covering topics such as nitrogen concentration and fertilizing frequency. He'll also demonstrate how to measure the pH in an orchid pot and how to use electrical conductivity measurements to monitor orchid nutrition. AtlOS members can bring plants to sell at the March meeting. Please remember that 10% of sales should be donated to the society. Cynorkis fastigiata Greengrowers at Rob Rinn’s house on March 18 Our first Greengrower’s visit of the year will be to Rob Rinn’s house. Please see page 4 for details. Inside This Issue Atlanta Orchid Society 2006 Officers…………………………………………..….…………… Page 2 Member Spotlight – Don & Mary Helen Reinhard.………………………...……....………….. Page 2 Events Out and About………………Dates for your Calendar…………...……….…….……… Page 3 Minutes of the February Meeting ….…….…...……….………….…………..………...….…. -

Systematics and Evolution of the Genus Pleurothallis R. Br
Systematics and evolution of the genus Pleurothallis R. Br. (Orchidaceae) in the Greater Antilles DISSERTATION zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) im Fach Biologie eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät I der Humboldt-Universität zu Berlin von Diplom-Biologe Hagen Stenzel geb. 05.10.1967 in Berlin Präsident der Humboldt-Universität zu Berlin Prof. Dr. J. Mlynek Dekan der Mathematisch-Naturwissenschaftlichen Fakultät I Prof. Dr. M. Linscheid Gutachter/in: 1. Prof. Dr. E. Köhler 2. HD Dr. H. Dietrich 3. Prof. Dr. J. Ackerman Tag der mündlichen Prüfung: 06.02.2004 Pleurothallis obliquipetala Acuña & Schweinf. Für Jakob und Julius, die nichts unversucht ließen, um das Zustandekommen dieser Arbeit zu verhindern. Zusammenfassung Die antillanische Flora ist eine der artenreichsten der Erde. Trotz jahrhundertelanger floristischer Forschung zeigen jüngere Studien, daß der Archipel noch immer weiße Flecken beherbergt. Das trifft besonders auf die Familie der Orchideen zu, deren letzte Bearbeitung für Cuba z.B. mehr als ein halbes Jahrhundert zurückliegt. Die vorliegende Arbeit basiert auf der lang ausstehenden Revision der Orchideengattung Pleurothallis R. Br. für die Flora de Cuba. Mittels weiterer morphologischer, palynologischer, molekulargenetischer, phytogeographischer und ökologischer Untersuchungen auch eines Florenteils der anderen Großen Antillen wird die Genese der antillanischen Pleurothallis-Flora rekonstruiert. Der Archipel umfaßt mehr als 70 Arten dieser Gattung, wobei die Zahlen auf den einzelnen Inseln sehr verschieden sind: Cuba besitzt 39, Jamaica 23, Hispaniola 40 und Puerto Rico 11 Spezies. Das Zentrum der Diversität liegt im montanen Dreieck Ost-Cuba – Jamaica – Hispaniola, einer Region, die 95 % der antillanischen Arten beherbergt, wovon 75% endemisch auf einer der Inseln sind. -

Habenaria Transvaalensis Schltr. and Habenaria Bonateoides M.Ponsie (Orchidaceae), Revised Descriptions and Distributional Records ⁎ M.E
South African Journal of Botany 73 (2007) 355–359 www.elsevier.com/locate/sajb Habenaria transvaalensis Schltr. and Habenaria bonateoides M.Ponsie (Orchidaceae), revised descriptions and distributional records ⁎ M.E. Ponsie , T.J. Edwards, S.D. Johnson School of Biological and Conservation Sciences, University of KwaZulu-Natal, Private Bag X01, Scottsville, Pietermaritzburg, 3209, South Africa Received 15 December 2006; received in revised form 26 January 2007; accepted 29 January 2007 Abstract Bonatea bracteata G.McDonald and McMurtry and Bonatea tentaculifera Summerh. were recently transferred to the genus Habenaria,as Habenaria transvaalensis Schltr. and Habenaria bonateoides M.Ponsie respectively. Until now H. bonateoides was known only from the holotype collected at Nairobi National Park in Kenya but new collections of the species from Tanzania expand its known range and suggest that sporadic populations may occur across a wider distribution. A full description, photographs and distributional data are provided. An amended description and distribution range of Habenaria transvaalensis is also provided to accommodate the inclusion of Bonatea bracteata within this species. © 2007 SAAB. Published by Elsevier B.V. All rights reserved. Keywords: Bonatea; Habenaria; Orchidaceae; Orchidoideae; Kenya; South Africa; Tanzania 1. Introduction In their revision of Bonatea, Ponsie et al. (2007b) transferred the two species B. bracteata G.McDonald and McMurtry and The status of Bonatea will remain controversial until a B. tentaculifera Summerh. to Habenaria. Here we provide broad-based revision of Habenaria is undertaken throughout additional critical distributional data that expand the ranges of its distribution. Molecular investigations focusing on Bonatea the respective species. Morphological data of specimens from (Ponsie et al., 2007a) have defined the genus as monophyletic this broadened distribution range necessitated amending exist- but were unable to address its taxonomic status in relation to ing descriptions. -

Partial Endoreplication Stimulates Diversification in the Species-Richest Lineage Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.12.091074; this version posted May 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Partial endoreplication stimulates diversification in the species-richest lineage of 2 orchids 1,2,6 1,3,6 1,4,5,6 1,6 3 Zuzana Chumová , Eliška Záveská , Jan Ponert , Philipp-André Schmidt , Pavel *,1,6 4 Trávníček 5 6 1Czech Academy of Sciences, Institute of Botany, Zámek 1, Průhonice CZ-25243, Czech Republic 7 2Department of Botany, Faculty of Science, Charles University, Benátská 2, Prague CZ-12801, Czech Republic 8 3Department of Botany, University of Innsbruck, Sternwartestraße 15, 6020 Innsbruck, Austria 9 4Prague Botanical Garden, Trojská 800/196, Prague CZ-17100, Czech Republic 10 5Department of Experimental Plant Biology, Faculty of Science, Charles University, Viničná 5, Prague CZ- 11 12844, Czech Republic 12 13 6equal contributions 14 *corresponding author: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.05.12.091074; this version posted May 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 15 Abstract 16 Some of the most burning questions in biology in recent years concern differential 17 diversification along the tree of life and its causes. -

Phylogenetic Analysis of Andinia (Pleurothallidinae; Orchidaceae) and a Systematic Re-Circumscription of the Genus
Phytotaxa 295 (2): 101–131 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2017 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.295.2.1 Phylogenetic analysis of Andinia (Pleurothallidinae; Orchidaceae) and a systematic re-circumscription of the genus MARK WILSON1, GRAHAM S. FRANK1, LOU JOST2, ALEC M. PRIDGEON3, SEBASTIAN VIEIRA-URIBE4,5 & ADAM P. KARREMANS6,7 1Department of Organismal Biology and Ecology, Colorado College, Colorado Springs, CO 8903, USA; e-mail: [email protected] 2Fundacion EcoMinga, Baños, Tungurahua, Ecuador. 3Royal Botanic Gardens, Kew, Richmond, Surrey, England. 4Grupo de Investigación en Orquídeas, Ecología y Sistemática Vegetal, Universidad Nacional, sede Palmira, Colombia. 5Sociedad Colombiana de Orquideología, AA. 4725 Medellín, Colombia. 6Lankester Botanical Garden, University of Costa Rica, P.O. Box 302-7050 Cartago, Costa Rica. 7Naturalis Biodiversity Center, Leiden, The Netherlands. Abstract Most of the species studied in this paper have previously been placed in either Pleurothallis or Lepanthes. However, at one time or another, members of the group have also been placed in the genera Andinia, Brachycladium, Lueranthos, Masdeval- liantha, Neooreophilus, Oreophilus, Penducella, Salpistele and Xenosia. Phylogenetic analyses of nuclear ITS and plastid matK sequences indicate that these species form a strongly supported clade that is only distantly related to Lepanthes and is distinct from Pleurothallis and Salpistele. Since this clade includes the type species of Andinia, A. dielsii, and it has taxo- nomic precedence over all other generic names belonging to this group, Andinia is re-circumscribed and expanded to include 72 species segregated into five subgenera: Aenigma, Andinia, Brachycladium, Masdevalliantha and Minuscula. -
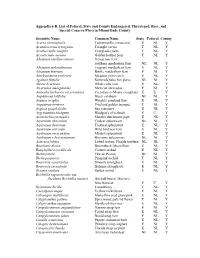
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

American· Orchid Society Bulletin
~e AMERICAN· ORCHID SOCIETY BULLETIN VOL. 1 JUNE,1932 No.1 . Cypripediu1% LawrenceanU11It PUBLISHED BY THE" TRUSTEES OF THE AMERICAN ORCHID SOCIETY THE AMERICAN ORCHID SOCIETY BULLETIN A Magazine Devoted to the Popularizing of Orchids and their Culture PRESENT ROLL OF OFFICERS' AND TRUSTEES April 29, 1932 OFFICERS President, F. E. DIXON, Secretary, DAVID LUMSDEN, 1411 Chestnut Street, Philadelphia, Pa. 115 Glenbrook Road, Bethesda, Md. Treasurer, WALTER H. JEWELL, New Rochelle, New York. VICE-PRESIDENTS OAKES AMES, MRS. WILLIAM K. DUPONT, 225 Bay State Road, Boston, Mass. Wilmington, Delaware. MRS. PIERRE S. DUPONT, JOSEPH E. WIDENER, Kennett S'quare, Pennsylvania. Elkins Park, Pennsylvania. GEORGE T. MOORE, Missouri Botankall Garden, St. Louis, Mo. HARRY G. HASKELL, 9044 duPont Building, Wilmington, Del. WILLIAM R. COE, The Chrysler Bldg., E. 42nd St., JAMES C. AUCHINCLOSS, New York, N. Y. 1 Wall Street, New York, N. Y. EDWIN S. WEBSTER, 49 Federa'l Street, Boston, Mass. TRUSTEES' Terms Expiring in 1933 GEORGE E. BALDWIN, JOSEPH MANDA, Mamaroneck, N ew York. 130-132 Main Street, W. Orange, N. J. J. J. MURDOCK, P ARMELY HERRICK, 35 Larchmont Avenue, Larchmont, N. Y. 720 Cuyahoga Bldg., Cleveland, Ohio. JOHN W. SLOTTER, Chadd's Ford, Pennsylvania. Terms Expiring in 1934 MRS. R. B. STRASSBURGER, OLIVER LINES, Gwynedd Valley, Pennsylvania. Ronaele Farms, Elkins Park, Penna. TOHN E. LAGER, DAVID H. HOLMES, Summit, N e.w J e.rsey. Bound Brook, New Jersey. ALBERT C. BURRAGE, JR., Ipswioh, Massachusetts. Terms Expiring in 1935 ERNEST B. DANE, W ALTER ARMACOST, 6 Beacon Street, Boston, Mass. Sawtelle, California. ROBERT H. ROLAND, W. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca.