Application of Azeotropic Distillation to the Separation of Olefins And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Centrifan PE Personal Evaporator User Manual
CentrifanTM PE Personal Evaporator User Manual (Shown – CentrifanTM – PE–T; Model 78-0070 with Circulator Coil Accessory) KD Scientific 84 October Hill Road Holliston, MA 01746 Phone: (508) 429-6809 Fax: (508) 893-0160 Part 78-8425 Manual www.kdscientific.com Notices: This system is covered by a limited lifetime warranty. A copy of the warranty is included with this manual. The operator is not required to perform routine maintenance, but procedures for disassembly and cleaning are as described herein. For cleaning procedures, refer to Chapter 4. All information in this manual is subject to change without notice and does not represent a commitment on the part of KD Scientific. The system and various components in the system are the subject of various pending patents. Modular SFC was acquired by Harvard Bioscience in May 2012. KD Scientific is a division of Harvard Bioscience. No part of this manual may be reproduced or transmitted in any form or by any means without the written permission of KD Scientific. All trademarks of other companies are acknowledged to the property of the respective owners. CentrifanTM is a trademark of KD Scientific. Printed in the United States of America. Warnings and Safety Precautions The following precautions should be followed to minimize the possibility of personal injury and/or damage to property while using the CentrifanTM PE. 1. Maintain a Well-Ventilated Laboratory Volatile organic solvents may be used in the system. Ensure that the laboratory is well ventilated so that a buildup of vaporized solvent cannot occur. 2. Ensure That Solvent Vapor Is Not Allowed To Escape Into The Laboratory Any organic solvent vapor must be vented to an external venting system so that it cannot collect in the laboratory. -

Escherichia Coli HD701
The Process Intensification of Biological Hydrogen Production by Escherichia coli HD701 By Michael Sulu A thesis submitted to The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Chemical Engineering College of Engineering and Physical Sciences The University of Birmingham November 2009 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Abstract Hydrogen is seen as a potential fuel for the future; its choice is driven by the increasing awareness of the necessity for clean fuel. Together with the simultaneous development of “green technologies” and sustainable development, a current goal is to convert waste to energy or to create energy from a renewable resource. Biological processing [of renewables] or bioremediation of waste to create hydrogen as a product fulfils this goal and, as such, is widely researched. In this work, an already established process, using a hydrogenase up‐regulated strain ‐ was characterised and the important process parameters were established. This bacterial strain has the potential for industrial‐scale hydrogen production from, for example, waste sugars. Previous work, repeated here, showed that hydrogen could be generated by E. -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Heterogeneous Azeotropic Distillation
PROSIMPLUS APPLICATION EXAMPLE HETEROGENEOUS AZEOTROPIC DISTILLATION EXAMPLE PURPOSE This example illustrates a high purity separation process of an azeotropic mixture (ethanol-water) through heterogeneous azeotropic distillation. This process includes distillation columns. Additionally these rigorous multi- stage separation modules are part of a recycling stream, demonstrating the efficiency of ProSimPlus convergence methods. Specifications are set on output streams in order to insure the required purity. This example illustrates the way to set "non-standard" specifications in the multi-stage separation modules. Three phase calculations (vapor-liquid- liquid) are done with the taken into account of possible liquid phase splitting. ACCESS Free-Internet Restricted to ProSim clients Restricted Confidential CORRESPONDING PROSIMPLUS FILE PSPS_EX_EN-Heterogeneous-Azeotropic-Distillation.pmp3 . Reader is reminded that this use case is only an example and should not be used for other purposes. Although this example is based on actual case it may not be considered as typical nor are the data used always the most accurate available. ProSim shall have no responsibility or liability for damages arising out of or related to the use of the results of calculations based on this example. Copyright © 2009 ProSim, Labège, France - All rights reserved www.prosim.net Heterogeneous azeotropic distillation Version: March, 2009 Page: 2 / 12 TABLE OF CONTENTS 1. PROCESS MODELING 3 1.1. Process description 3 1.2. Process flowsheet 5 1.3. Specifications 6 1.4. Components 6 1.5. Thermodynamic model 7 1.6. Operating conditions 7 1.7. "Hints and Tips" 9 2. RESULTS 9 2.1. Comments on results 9 2.2. Mass and energy balances 10 2.3. -

Distillation Accessscience from McgrawHill Education
6/19/2017 Distillation AccessScience from McGrawHill Education (http://www.accessscience.com/) Distillation Article by: King, C. Judson University of California, Berkeley, California. Last updated: 2014 DOI: https://doi.org/10.1036/10978542.201100 (https://doi.org/10.1036/10978542.201100) Content Hide Simple distillations Fractional distillation Vaporliquid equilibria Distillation pressure Molecular distillation Extractive and azeotropic distillation Enhancing energy efficiency Computational methods Stage efficiency Links to Primary Literature Additional Readings A method for separating homogeneous mixtures based upon equilibration of liquid and vapor phases. Substances that differ in volatility appear in different proportions in vapor and liquid phases at equilibrium with one another. Thus, vaporizing part of a volatile liquid produces vapor and liquid products that differ in composition. This outcome constitutes a separation among the components in the original liquid. Through appropriate configurations of repeated vaporliquid contactings, the degree of separation among components differing in volatility can be increased manyfold. See also: Phase equilibrium (/content/phaseequilibrium/505500) Distillation is by far the most common method of separation in the petroleum, natural gas, and petrochemical industries. Its many applications in other industries include air fractionation, solvent recovery and recycling, separation of light isotopes such as hydrogen and deuterium, and production of alcoholic beverages, flavors, fatty acids, and food oils. Simple distillations The two most elementary forms of distillation are a continuous equilibrium distillation and a simple batch distillation (Fig. 1). http://www.accessscience.com/content/distillation/201100 1/10 6/19/2017 Distillation AccessScience from McGrawHill Education Fig. 1 Simple distillations. (a) Continuous equilibrium distillation. -

Tissue Free Water Tritium Separation from Foodstuffs by Azeotropic Distillation
140 SK98K0361 21st RHDJasna podChopkom TISSUE FREE WATER TRITIUM SEPARATION FROM FOODSTUFFS BY AZEOTROPIC DISTILLATION Florentina Constantin, Ariadna Ciubotaru, DecebalPopa Inspectorate of Public Health of Bucharest, Romania 1. INTRODUCTION The actual tritium content in the environment is due to both natural production by cosmic ray interactions in the upper atmosphere and to varying amount of man- made tritium. Tritium, the radioactive isotope of hydrogen, is one of the most important radionuclide released to the environment during the normal operation of a typical nuclear power reactor. Tritiated water constitutes almost the entire radioactivity in liquid waste discharged to the environment from heavy water reactors. Therefore, monitoring tritium in air, precipitation, drinking water and vegetation is necessary for assessing the environmental impact of tritium releases in gaseous and liquid effluents from nuclear plants. The use for agricultural purposes of river water receiving releases of tritium, results in contamination of irrigated crops and then in the contamination of the food chain. ? Tritium is everywhere in living matter, has a long physical half-time (T= 12.26 y) and is known to follow protium pathways in biological material. Tritium in liquid or solid biota samples can not be easily determined quantitatively by any kind of nondestructive analysis because its beta radiation energy (E =18 keV) is so weak that the most part is absorbed in the sample. Therefore, most non-aqueous samples must be treated chemically or physically to be suitable for counting. The choice of preparation method will depend on the type of samples, number and quantities of samples to be processed and availability of measurement equipment. -
Grout Gas Generation Test Plan ECN No
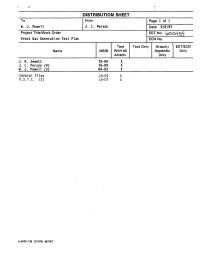
DISTRIBUTION SHEET To From Page 1 of 1 - W. J. Powell J. C. Person Date 9/8/93 Project Title/Work Order EDTNo. (pOo45^j[ Grout Gas Generation Test Plan ECN No. Text Text Only Attach./ EDT/ECN Name MSIN With All Appendix Only Attach. Only J. R. Jewett T6-09 X J. C. Person (4) T6-09 X W. J. Powell (3) R4-03 X Central Files L8-04 X 0.S.T.I. (2) L8-07 X A-6000-135 (01/93) WEF067 Page 1 of | ai p7 4QQC ENGINEERING DATA TRANSMITTAL I.EDT 600459 2. To: (Receiving Organization) 3. From: (Originating Organization) 4. Related EDT No.: Grout Technology Process Chemistry Laboratory N/A S. Proj./Prog./Dept./Div.: 6. Cog. Engr.: 7. Purchase Order No.: WM/Grout/PAL/FO J. C. Person _N/A_ 8. Originator Remarks: 9. Equip./Component No. N/A 10. Systea/Bldg./Facility: N/A 11. Receiver Remarks: 12. Major Assm. Dwg. No. 13. Permit/Permit Application No.: 14. Required Response Date: 15. DATA TRANSMITTED (F) (G) (H) (I) (A| (C) (D) Reason Origi• Receiv• (E) Title or Description of Data Impact Item Sheet Rev. for nator er (B) Document/Drawing No. Transmitted Level No. No. No. Trans• Dispo• Dispo• mittal sition sition 1. WHCSD-WM-TP-180 Grout Gas Generation 3Q Test Plan 16. KEY Impact Level (F) Reason for Transmittal (G) Disposition (H) & (I) 1, 2, 3, or 4 (see 1. Approval 4. Review 1. Approved 4. Reviewed no/comment MRP 5.431 3Q 2. Release 5. Post-Review 2. Approved w/comment 5. -

Comparison of Complete Extractive and Azeotropic Distillation Processes for Anhydrous Ethanol Production Using Aspen Plustm Simu
43 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 80, 2020 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Eliseo Maria Ranzi, Rubens Maciel Filho Copyright © 2020, AIDIC Servizi S.r.l. DOI: 10.3303/CET2080008 ISBN 978-88-95608-78-5; ISSN 2283-9216 Comparison of Complete Extractive and Azeotropic Distillation Processes for Anhydrous Ethanol Production using Aspen PlusTM Simulator Nahieh T. Miranda*, Rubens Maciel Filho, Maria Regina Wolf Maciel Laboratory of Optimization, Design, and Advanced Control (LOPCA)/Laboratory of Development of Separation Processes (LDPS), School of Chemical Engineering, University of Campinas, Brazil. [email protected] Performance of the extractive and azeotropic distillation processes using ethylene glycol and cyclohexane as solvents, respectively, for anhydrous ethanol production, were investigated using 02 RadFrac columns each and solvent recycling streams via simulation in Aspen Plus™ Simulator. Both operate at 1 atm, and feed flow -1 rate equals to 100 kmol.h (ethanol – 0.896 and water – 0.104 in mole fractions at the azeotropic point). The NRTL-RK was the model used for extractive and azeotropic distillations. The anhydrous ethanol purity from top of the 1st column of the extractive distillation (22 stages) was 99.50 % (on a mole basis) and water with nd st 99.20 % of purity at the top of the 2 column. On the other hand, in the azeotropic distillation, the 1 column (30 stages) had a bottom product of anhydrous ethanol purity of 99.99 % and water with 99.99 % of purity in the 2nd column, also at the bottom. Both processes produced anhydrous ethanol with a high-grade purity required by the standard norms ASTM D4806, EN 15376, and ANP 36. -

Partial Separation of an Azeotropic Mixture of Hydrogen Chloride and Water and Copper (II) Chloride Recovery for Optimization of the Copper-Chlorine Cycle
Partial Separation of an Azeotropic Mixture of Hydrogen Chloride and Water and Copper (II) Chloride Recovery for Optimization of the Copper-Chlorine Cycle by Matthew P. Lescisin A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Applied Science in The Faculty of Engineering and Applied Science Mechanical Engineering University of Ontario Institute of Technology September 2017 © Matthew P. Lescisin, 2017 Contents Contents ii Abstract v Acknowledgments vi List of Figures vii List of Tables viii Nomenclature ix Quantities...................................... ix Greek Letters.................................... x Subscripts...................................... xi Acronyms...................................... xi 1 Introduction1 2 Background4 2.1 Boiling in the Subcooled Liquid Region .................. 4 2.2 Vapor-Liquid Equilibrium (VLE)...................... 5 2.3 Phase Diagrams................................ 5 2.3.1 Tie-Lines ............................... 6 2.4 Distillation .................................. 6 2.5 Azeotropes .................................. 8 3 Literature Review9 3.1 Azeotropic Distillation............................ 9 3.2 Extractive Distillation............................ 10 3.3 Pressure-Swing Distillation .......................... 11 3.4 Batch Mode.................................. 13 3.5 Heat-Integrated Distillation Columns.................... 13 3.5.1 Heat-Integrated PSD......................... 14 ii CONTENTS iii 3.6 Reflux Ratio ................................. 15 -

KNF Rotary Evaporator Brochure
RC 900 AND RC 600 ROTARY EVAPORATORS. INSPIRINGLY EASY TO USE. SYSTEMS EXPERTISE BENEFIT FROM EXPERT KNOWLEDGE. ROTARY EVAPORATION TAILORED TO PRACTICAL NEEDS. Under the spotlight at KNF: What aspects are really key What makes KNF’s rotary evaporators stand out? For to rotary evaporation in everyday lab practice? What is years we have offered the SC 920 vacuum pump system, needed to guarantee simple, economical and reliable a much sought-after laboratory device that works side by processes day in day out? These are the questions we side with distilling apparatus. Simple handling, a small used to guide us when developing and implementing the footprint and whisper quiet operation are the KNF RC 900 and the new RC 600. We became involved in daily strengths that set the SC 920 apart. lab work. We asked lab technicians what they wished for, enlisted experts to perform tests, and incorporated their Its success has encouraged us to broaden our systems suggestions. approach to create a perfectly balanced package comprising The result? KNF rotary evaporators designed to impress a rotary evaporator, vacuum pump system, and chiller. thanks to their distinct handling advantages, clever Curious? Then discover KNF’s rotary evaporators and functional details and well thought out safety features. their advantages today! EASY TO USE | CLEVER FUNCTIONAL DETAILS | WELL THOUGHT OUT SAFETY FEATURES RC 900. SUPERIOR PERFORMANCE SYSTEM. RC 600. DUAL EVAPORATION FOR ACADEMIA LABS. Rotary evaporator, vacuum pump system, and chiller as a perfectly System packages to suit different budget conditions are available – coordinated system. e.g., one comprising a vacuum pump system to simultaneously and independently assist two rotary evaporators. -

Studying the Effect of Mixing Miscible Hydrocarbon Components on the Multi-Stage Distillation Operation Compared with Single Component Distillation
STUDYING THE EFFECT OF MIXING MISCIBLE HYDROCARBON COMPONENTS ON THE MULTI-STAGE DISTILLATION OPERATION COMPARED WITH SINGLE COMPONENT DISTILLATION MOHD NOR FIRDAUS BIN MOHD SALLEH A thesis submitted in fulfilment of the Requirement for the award of the degree of Bachelor of Chemical Engineering (Gas Technology) Faculty of Chemical and Natural Resources Engineering University Malaysia Pahang (UMP) APRIL 2008 ii I declare that this thesis entitled ‘Effect of mixing miscible hydrocarbon component on the multi-stage distillation operation compared with single component’ is the results of my own research except as cited in the references. The thesis has not been accepted for any degree and is not concurrently submitted candidature of any degree. Signature : ……………………………………… Name : MOHD NOR FIRDAUS BIN MOHD SALLEH Date : 30 th APRIL 2008 iii Special dedicated to my beloved father and mother and my whole family members iv ACKNOWLEDGEMENT Alhamdulillah all praises be to almighty Allah who made it possible for me to done this thesis by time. First of all, I would like to express my appreciation especially to my loving caring parent, Mr. Mohd Salleh bin Daud and Madam Rahah binti Md Salleh and rest of my family members who are very supportive morally to whatever good things that I have involve and done all these years. Secondly I am greatly indebted to final year project supervisor Doctor Hayder A. Abdul Bari who was very kind to accept me as his supervise student and allowed me to work with him until this project is done. He greatly influenced, guidance, encouragement, comments and motivated me to explore distillation process in depth. -

FEI Tecnai 12 ('T12') 120 Kv
Ver. 2017-07-07 Emergency Information: 1. Medical Emergencies: Contact 911 and McGill Security 514.398.3000 2. Leave TEM as is. Do NOT shut down the vacuum system. 3. If possible, turn off High Tension and Close Column Valve. 4. Exit the Room/Building. Emergency Contact Information: • Jeannie Mui – EM Coordinator: Office 514.398.6329; Email: [email protected] • S. Kelly Sears – Facility Manager: Cell 514.576.1926; Email: [email protected] • Joaquin Ortega – Director: Office 514.398.6348; [email protected] FEI Tecnai 12 (‘T12’) 120 kV TEM Simplified Operating Manual (Prepared by S Kelly Sears and Joaquin Ortega) Safety • Do NOT wander behind the microscope or step on cables. • Do NOT touch the sample rod with bare hands below the black O-ring. • There are two computers on the T12 TEM: 1. Microscope PC (MPC) that controls the TEM • Note: Under no circumstances should a user insert a USB device into the MPC. 2. Support PC (SPC) to store and retrieve images (on the desk to the left of the TEM). Protective Equipment • Nitrile gloves for handling sample holder and safety glasses for filling liquid nitrogen dewar. I. Microscope Start Up Note: Your PC desktop may not appear exactly as shown in the images in this manual. a. VERIFY the Red On/Off button and the White Vac. and HT buttons on the right console are lit (currently NOT WORKING). b. LOG IN to the MPC with the username and password created during your training session. After logging in, VERIFY the microscope icon on the right side of the bottom task bar is present and green.