Indian Pharmaceutical Industry 2021: Future Is Now
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Do We Know About India's Covaxin Vaccine?
FEATURE Tamil Nadu, India COVID-19 VACCINES [email protected] BMJ: first published as 10.1136/bmj.n997 on 20 April 2021. Downloaded from Cite this as: BMJ 2021;373:n997 http://dx.doi.org/10.1136/bmj.n997 What do we know about India’s Covaxin vaccine? Published: 20 April 2021 India has rapidly approved and rolled out Covaxin, its own covid-19 vaccine. Kamala Thiagarajan examines what we know so far. Kamala Thiagarajan freelance journalist Who developed Covaxin? cheapest purchased by any country in the world at 206 rupees per shot for the 5.5 million doses the Covaxin was developed by Indian pharmaceutical government currently has on order. The government company Bharat Biotech in collaboration with the has capped the price of the vaccine sold in the private Indian Council of Medical Research, a government market, with private hospitals able to charge up to funded biomedical research institute, and its 250 rupees.13 subsidiary the National Institute of Virology. Covaxin does not require storage at sub-zero Bharat Biotech has brought to market 16 original temperatures, which would be hard to maintain in vaccines, including for rotavirus, hepatitis B, Zika India’s climate and with the frequent power cuts in virus, and chikungunya.1 The company reportedly rural areas. Covaxin is available in multi-dose vials spent $60-$70m (£43-£50m; €50-€58m) developing and is stable at the 2-8°C that ordinary refrigeration Covaxin.2 can achieve. How does Covaxin work? Bharat Biotech says it has a stockpile of 20 million The vaccine is similar to CoronaVac (the Chinese doses of Covaxin for India and is in the process of vaccine developed by Sinovac)3 in that it uses a manufacturing 700 million doses at its four facilities complete infective SARS-CoV-2 viral particle in two cities by the end of the year. -

Towards a New Dawn Lr
lR;eso t;rs Towards a New Dawn lR;eso t;rs ANNUAL REPORT 2009-10 Towards a New Dawn MINISTRY OF WOMEN AND CHILD DEVELOPMENT Government of India CONTENTS Page No. Chapter 1. Introduction 1 Chapter 2. Women Development 11 Chapter 3. Child Development 25 Chapter 4. Child Protection and Welfare 43 Chapter 5. Gender Budgeting 53 Chapter 6 Other Programmes and Activities 63 Chapter 7. Food and Nutrition Board 71 Chapter 8. National Institute of Public Cooperation 79 and Child Development Chapter 9. Central Social Welfare Board 91 Chapter 10. National Commission for Women 99 Chapter 11. Rashtriya Mahila Kosh 109 Chapter 12. National Commission for Protection of 119 Child Rights Chapter 13. Central Adoption Resource Authority 127 Annexures 133 1 Introduction Towards a New Dawn Chapter 1 Introduction 1.1 The Ministry of Women and Child and well nurtured children with full opportunities Development, Government of India, came into for their growth and development in an existence as a separate Ministry w.e.f. 30th environment free from exploitation. January 2006. It is the nodal Ministry for all Mission matters pertaining to development of women and children who constitute 71.14% of the 1.3 In pursuance of the vision, the Mission of the country's population, as per the 2001 Census. Ministry of Women and Child Development is to: Vision (i) promote social and economic empowerment of women through cross-cutting policies and 1.2 The vision of the Ministry of Women programmes, mainstream gender concerns, and Child Development is to have empowered create awareness about their rights and women living with dignity and contributing as facilitate institutional and legislative support equal partners towards the development of the for enabling them to develop to their full country in an environment free from violence potential. -

Indian-American Kids Sweep Spelling and Geography Bees
JUNE, 2012 CLEVELAND, OHIO PRICELESS–ONE COPY PER FAMILY GENERAL DENTIST ™ Cosmetic Dentistry INDIA GROCERS Emergencies & New Patients Welcome 6855 W. 130th St., Parma Hts. OH 44130 Early AM, Evening & Weekend Hours Most Dental Insurance Plans Phone 440-885-0215 Medicaid, Caresource Accepted All Indian Groceries ~ Fresh Vegetables RootCanal, Dentures, India (We accept Ohio Food Stamps) Dr. Shyam Sharma, DDS Bleaching, Crowns, Other Ohio Location: Columbus 614.798.9331 440-826-0423 Bridges INTERNATIONAL Middleburg Hts., 18660 Bagley Rd., Suite 304 (Bldg 2) Voice of Asian-Indian Americans www.allindiagrocers.com An Independent and the Largest Newspaper of the Asian-Indian Community in Ohio 85 Percent I N S I D E Indian-Americans Bulletinboard ........... 2 Support Obama, Follow Your Dreams .. 5 India Quiz ............. 5 Says Survey Community News ... 6–7 WASHINGTON: The Indian- Art & Culture ......... 8-9 American community has News from India ..... 10 come out in strong support News from US .. ...... 11 of US President Barack Useful Info ............... 12 Obama , who kicked off his Humor, Riddles ........ 13 re-election campaign with Immigration ............ 15 two rallies in Ohio and Virginia last month, with an overwhelming 85 per cent of Tips for Handling them favoring a second term Unwanted for him. About 85 per cent of the Telemarketing Calls Indian-Americans support –Page 12 Obama for a second term, according to a latest survey conducted by Lake NRO, NRE, FCNR Research Partners, a DC- based political consultancy Term Deposits: firm, with APIAVote. APIA Which One to Pick? stands for Asian American –Page 4 Pacific Islander. Asian Children perform a dance at the Cleveland Asian Festival May 19, 2012 “President Obama was strongest among Indian- American voters, leading Pro-Women Divorce Mitt Romney by a margin of Clause in India’s Law 76 to eight per cent in the –Page 10 poll, and weakest among Filipino Americans, where the vote was 57 per cent to Cleveland’s Neha is 20 per cent. -

Proud of Sikh Heritage
Citation of Qaumi Seva Award presented to Prime Minister Narendra Modi by SGPC on the historic occasion of the inauguration of Sri Kartarpur Sahib corridor on 9th November, 2019 QAUMI SEVA AWARD On the auspicious occasion of 550th Prakash Purab of Sachey Patshah Satguru (True Emperor True Teacher) Nanak Dev Ji Maharaj, the Sikh Qaum (community) has been blessed by Akal Purakh (Eternal Entity) and the great Guru Sahibs on this historic occasion; the daily prayers of all the sangat (Congregation), belonging to every nook and corner of the world for decades are being accepted in the Dargah of Akal Purakh (The Almighty God’s Court). As a result and as a first step, a corridor connecting Dera Baba Nanak Sahib and the shrines of Guru Nanak Patshah’s life at Kartarpur Sahib (Pakistan) has been opened for the sangat. On the 550th birth anniversary of Satguru Sachey Patshah Ji, what greater divine gift could the Sikh Sangat have received than for a head of the country to become the Messiah and show political, administrative and diplomatic courage for fulfilling this wish of the Sikh community. It is only by the grace of the Guru that the joy of opening of this corridor of faith, belief and love for humanity has been bestowed upon the person, who himself is deeply in love with Sikhism and greatly devoted to the Guru’s feet. An example of this devotion is the unparalleled contribution of Prime Minister Narendra Modi in celebrating the 550th birth anniversary of Guru Maharaj, including the opening of Sri Kartarpur Sahib corridor and making Sultanpur Lodhi, the first karambhumi of Guru Sahib, a state-of-the- art smart city. -

Output Outcome Framework for Schemes 2018-2019 Demand No
PREFACE Major Expenditure Reforms have been undertaken by the Government over the last two-three years. This not only includes simplification of appraisal and approval processes, but also structural changes in the process of budget making itself like doing away with Plan Non-plan distinction. As a result,the cost-centres are being treated in an integrated manner, within only the statutory revenue capital framework. This enables another major structural reform, which is to bring the public schemes and projects under a monitorable Output-Outcome framework. Since 2017-18, in addition to the financial outlays of schemes of the Ministries being indicated in the Budget document, the expected outputs and outcomes of the schemes were also prepared and presented separately by each Ministry in the form of Outcome Budget. T h e s e Outlays, Outputs and Outcomes are being presented to the Parliament in measurable terms, bringing-in greater accountability for the agencies involved in the execution of government schemes and projects. utlay is the amount that is provided for a given scheme or project in the Budget; while Outpu refers to the direct and measurable product of program activities, often expressed in physical terms or units. utcome are the collective results or qualitative improvements brought about in the delivery of these services, often expressed in terms of improvements over ex-ante or earlier indicators and benchmarks. From the last year s budget, it was decided that the output and outcomes of the schemes of 68 Ministries and Departments would be available along with the financial outlays as a part of the Budget documents, so that clearly defined objectives and goals for each scheme can be seen by all. -

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

ED054708.Pdf
DOCUMENT RESUME ED 054 708 HE 002 349 AUTHOR Spencer Richard E.; Awe, Ruth TITLE International Educational Exchange. P. Bibliography. INSTITUTION Institute of International Education, New York N.Y. PUB DATE 70 NOTE 158p- AVAILABLE FROM Institute of Internationa Education, 809 United Nations Plaza, New York, New York 10017 EDRS PRICE MF-S0.65 HC-$6.58 DESC IPTORS *Bibliographies; *Exchange Programs; *Foreign Students; *International Education; International Programs; *Research; Student Exchange Programs; Teacher Exchange Programs ABSTRACT This bibliography was undertaken to facilitate and encourage further research in international education. Sources of the data include library reference works, University Microfilms containing PhD dissertations, US government agencies, foundations and universities. Entries include publications on the International Exchange of Students, Teachers and Specialists and cover: selection, admissions, orientation, scholarships, grants, foreign student advisors, attitudes, and adjustment, hospitality of host country, community relations, academic achievement, returnees, follow-up evaluations, brain drain, professional educators, specialists, US nationals abroad, foreign students and visitors in the US, personnel and program interchanges, immigration policies, international activities of US universities. Entries on.Educational Curriculum cover: English as a second language, linguistics and other languages, courses of study. The last 3 sectional entries are: General Works on International Educational and Cultural Exchange; Cross-Cultural and Psychological Studies Relevant to Educational EX hange; and Bibliographies. (JS) o;c;lopD10-01.0 1 2405-010° w,64.'<cm -10 2B164. 01-0122 1.roz1;x2 .clito ccrupw00 -p 44u2u7LE°- 01-:<-,-.1-01wouuxoctzio 0014.0) 0 MO 'W 0042MOZ WICL,TA° 3 mulwan. 411 :IZI01/1°4 t4. INTERNATIONAL EDUCATIONAL EXCHANGE -4- a)A BIBLIOGRAPHY 4:3 by Richard E. -

Pharmaceutical Cluster in Andhra Pradesh
Pharmaceutical Cluster in Andhra Pradesh Microeconomics of Competitiveness Final Project Harvard Business School Helene Herve | Lhakpa Bhuti | Saurabh Agarwal | Sonny Kushwaha | Akbar Causer May 2013 Table of Contents 1 Executive Summary ............................................................................................................................ 3 2 Introduction to India ........................................................................................................................... 4 2.1 History and Political Climate ....................................................................................................... 5 2.2 Competitive Positioning of India ................................................................................................. 6 2.2.1 Endowments .......................................................................................................................... 6 2.2.2 Economic Performance To-Date and Macroeconomic Policy .............................................. 7 2.2.3 Summary of Export Clusters ................................................................................................. 9 2.2.4 Social Infrastructure and Political Institutions .................................................................... 10 2.2.5 India Diamond .................................................................................................................... 11 3 Andhra Pradesh ................................................................................................................................ -
Nifty Poised to Open Gap up on Strong Payroll Data
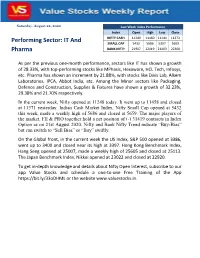
Saturday, August 22, 2020 Last Week Index Performance Index Open High Low Close NIFTY CASH 11249 11460 11144 11372 Performing Sector: IT And SMALL CAP 5432 5686 5357 5659 Pharma BANK NIFTY 21907 22419 21403 22300 As per the previous one-month performance, sectors like IT has shown a growth of 28.33%, with top-performing stocks like MPhasis, Hexaware, HCL Tech, Infosys, etc. Pharma has shown an increment by 21.88%, with stocks like Divis Lab, Alkem Laboratories, IPCA, Abbot India, etc. Among the Minor sectors like Packaging, Defence and Construction, Supplies & Fixtures have shown a growth of 32.23%, 29.38% and 21.70% respectively. In the current week, Nifty opened at 11248 today. It went up to 11458 and closed at 11371 yesterday. Indian Cash Market Index, Nifty Small Cap opened at 5432 this week, made a weekly high of 5686 and closed at 5659. The major players of the market, FII & PRO together hold a net position of (-) 31419 contracts in Index Option as on 21st August 2020. Nifty and Bank Nifty Trend indicate “Buy-Bias” but can switch to “Sell Bias” or “Buy” swiftly. On the Global front, in the current week the US Index, S&P 500 opened at 3386, went up to 3400 and closed near its high at 3397. Hong Kong Benchmark Index, Hang Seng opened at 25007, made a weekly high of 25605 and closed at 25113. The Japan Benchmark Index, Nikkei opened at 23022 and closed at 22920. To get in-depth knowledge and details about Nifty Open Interest, subscribe to our app Value Stocks and schedule a one-to-one Free Training of the App https://bit.ly/33oDHML or the website www.valuestocks.in. -

Partner with India
PARTNER WITH INDIA Powering India’s vision through reforms Prime Minister Shri Narendra Modi has laid Economic down India’s vision to become a US$5 trillion economy by 2025. India aims to accelerate its economic growth Snapshot and sustain a real GDP growth rate of 8%. The government continues to push for transformative reforms to attract more investments and make India an economic powerhouse across Asia-Pacific and beyond. 2 Economic Performance 2019-20 6.9% average economic growth 6% - 6.5% economic growth 5% economic growth estimated for financial reported by India over last five projected for financial year 2020-21 year 2019-20 ending March 31, 2020 financial years starting April 1, 2020 15% jump in FDI inflow to 12.7% rise posted in imports US$5 trn economy targeted by US$26.1bn during H1 2019-20, (manufactured), and 8.6% for total strengthening Indian market and export led by services and ITC sectors merchandise growth 13.4% rise posted in exports 0.7% rise in trade surplus/year (manufactured), and 10.9% for (manufactured), 2.3% rise for total total merchandise merchandise 3rd rank globally for India in number of new firms created, as per the World Bank Economic Performance 2019-20 Gross GST monthly collections Reforms undertaken during crossed Rs.1trn-mark 5 times 2019-20 to boost investment, US$38.4b in during April-Dec 2019 consumption and exports: remittances from ● Speeding up the insolvency overseas Indians Entrepreneurship promotion resolution process under the aimed to fuel productivity IBC in H1 2019-20; or growth and wealth -

Vaccine Matri: a New Way of Diplomacy
AN EXPLORATORY STUDY OF ROLE OF INDIA IN VACCINE DIPLOMACY FOR COVID-19 PANDEMIC ERA Turkish Online Journal of Qualitative Inquiry (TOJQI) Volume 12, Issue 3, June 2021:652- 665 Research Article An Exploratory Study Of Role Of India In Vaccine Diplomacy For Covid-19 Pandemic Era Dr. Saroj Choudhary1, Dr Sanjiv Singh Bhadauria2, Mr Abhinav Upadhyay3, Dr Sandeep Kulshrestha4 Abstract Coronavirus is spread in end of December, 2019 in Wuhan and in March, 2020 World Health Organization declared it as a pandemic. Coronavirus affect world in many ways like socially, economically and in many other. In early January, 2021 India make their vaccine and not only vaccinated their own people but also help other countries and provide vaccine to their neighbouring countries on grant and commercial basis and this will help India to make their reputation in world platform. In this paper, Vaccine Maîtri an initiative by India to provide vaccine to their neighbouring countries and also other needy countries and how this will enhance the image of India in world stage. Keywords: Coronavirus; COVID-19; vaccine maîtri; vaccine; India;government; WHO. VACCINE MATRI: A NEW WAY OF DIPLOMACY COVID-19 is a major global public health challenge, and in many countries it has created a serious social, economic, and political crisis. The numbers involved are staggering, whether they refer to infection and death, the rate of public health measures such as travel restrictions, or the economic consequences of unemployment and public sector spending. All economies are placed in drug-induced comas, the complex public health systems have become increasingly prevalent in levels of public adherence or surprising disobedience, and health care systems and provinces are being tested by many who have never seen it. -

Indian Students, 'India House'
Wesleyan University The Honors College Empire and Assassination: Indian Students, ‘India House’, and Information Gathering in Great Britain, 1898-1911 by Paul Schaffel Class of 2012 A thesis submitted to the faculty of Wesleyan University in partial fulfillment of the requirements for the Degree of Bachelor of Arts with Departmental Honors in History Middletown, Connecticut April, 2012 2 Table Of Contents A Note on India Office Records.............................................................................................3 Acknowledgements ...................................................................................................................4 Introduction-A Dynamic Relationship: Indian Students & the British Empire.....5 Separate Spheres on a Collision Course.................................................................................6 Internal Confusion ....................................................................................................................9 Outline...................................................................................................................................... 12 Previous Scholarship.............................................................................................................. 14 I. Indian Students & India House......................................................................... 17 Setting the Stage: Early Indian Student Arrivals in Britain .............................................. 19 Indian Student Groups .........................................................................................................