Petrogenetic Processes Characterizing the Mount Bachelor, Oregon Magmatic System: Open- Versus Closed-System Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volcanic Vistas Discover National Forests in Central Oregon Summer 2009 Celebrating the Re-Opening of Lava Lands Visitor Center Inside
Volcanic Vistas Discover National Forests in Central Oregon Summer 2009 Celebrating the re-opening of Lava Lands Visitor Center Inside.... Be Safe! 2 LAWRENCE A. CHITWOOD Go To Special Places 3 EXHIBIT HALL Lava Lands Visitor Center 4-5 DEDICATED MAY 30, 2009 Experience Today 6 For a Better Tomorrow 7 The Exhibit Hall at Lava Lands Visitor Center is dedicated in memory of Explore Newberry Volcano 8-9 Larry Chitwood with deep gratitude for his significant contributions enlightening many students of the landscape now and in the future. Forest Restoration 10 Discover the Natural World 11-13 Lawrence A. Chitwood Discovery in the Kids Corner 14 (August 4, 1942 - January 4, 2008) Take the Road Less Traveled 15 Larry was a geologist for the Deschutes National Forest from 1972 until his Get High on Nature 16 retirement in June 2007. Larry was deeply involved in the creation of Newberry National Volcanic Monument and with the exhibits dedicated in 2009 at Lava Lands What's Your Interest? Visitor Center. He was well known throughout the The Deschutes and Ochoco National Forests are a recre- geologic and scientific communities for his enthusiastic support for those wishing ation haven. There are 2.5 million acres of forest including to learn more about Central Oregon. seven wilderness areas comprising 200,000 acres, six rivers, Larry was a gifted storyteller and an ever- 157 lakes and reservoirs, approximately 1,600 miles of trails, flowing source of knowledge. Lava Lands Visitor Center and the unique landscape of Newberry National Volcanic Monument. Explore snow- capped mountains or splash through whitewater rapids; there is something for everyone. -

A Tale of Three Sisters: Reconstructing the Holocene Glacial History and Paleoclimate Record at Three Sisters Volcanoes, Oregon, United States
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 2005 A Tale of Three Sisters: Reconstructing the Holocene glacial history and paleoclimate record at Three Sisters Volcanoes, Oregon, United States Shaun Andrew Marcott Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Geology Commons, and the Glaciology Commons Let us know how access to this document benefits ou.y Recommended Citation Marcott, Shaun Andrew, "A Tale of Three Sisters: Reconstructing the Holocene glacial history and paleoclimate record at Three Sisters Volcanoes, Oregon, United States" (2005). Dissertations and Theses. Paper 3386. https://doi.org/10.15760/etd.5275 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. THESIS APPROVAL The abstract and thesis of Shaun Andrew Marcott for the Master of Science in Geology were presented August II, 2005, and accepted by the thesis committee and the department. COMMITTEE APPROVALS: (Z}) Representative of the Office of Graduate Studies DEPARTMENT APPROVAL: MIchael L. Cummings, Chair Department of Geology ( ABSTRACT An abstract of the thesis of Shaun Andrew Marcott for the Master of Science in Geology presented August II, 2005. Title: A Tale of Three Sisters: Reconstructing the Holocene glacial history and paleoclimate record at Three Sisters Volcanoes, Oregon, United States. At least four glacial stands occurred since 6.5 ka B.P. based on moraines located on the eastern flanks of the Three Sisters Volcanoes and the northern flanks of Broken Top Mountain in the Central Oregon Cascades. -

The Plumbing Systems and Parental Magma Compositions Of
THE PLUMBING SYSTEMS AND PARENTAL MAGMA COMPOSITIONS OF SHIELD VOLCANOES IN THE CENTRAL OREGON HIGH CASCADES AS INFERRED FROM MELT INCLUSION DATA by STANLEY PAUL MORDENSKY II A THESIS Presented to the Department of Geological Sciences and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Master of Science September 2012 THESIS APPROVAL PAGE Student: Stanley Paul Mordensky II Title: The Plumbing Systems and Parental Magma Compositions of Shield Volcanoes in the Central Oregon High Cascades as Inferred from Melt Inclusion Data This thesis has been accepted and approved in partial fulfillment of the requirements for the Master of Science degree in the Department of Geological Sciences by: Dr. Paul Wallace Chair Dr. Ilya Bindeman Member Dr. Katharine Cashman Member Dr. Dana Johnston Member and Kimberly Andrews Espy Vice President for Research & Innovation/Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded September 2012 ii © 2012 Stanley Paul Mordensky II iii THESIS ABSTRACT Stanley Paul Mordensky II Master of Science Department of Geological Sciences September 2012 Title: The Plumbing Systems and Parental Magma Compositions of Shield Volcanoes in the Central Oregon High Cascades as Inferred from Melt Inclusion Data Long-lived and short-lived volcanic vents often form in close proximity to one another. However, the processes that distinguish between these volcano types remain unknown. Here, I investigate the differences of long-lived (shield volcano) and short- lived (cinder cone) magmatic systems using two approaches. In the first, I use melt inclusion volatile contents for shield volcanoes and compare them to published data for cinder cones to investigate how shallow magma storage conditions differ between the two vent types in the Oregon Cascades. -

DIAMOND PEAKS Information Guide
DIAMOND PEAKS Information Guide COMMUNITY: Pristine one plus acre dream sites located in Southern Central Oregon near the Diamond Peak Wilderness Area and only minutes from Crescent Lake, Odell Lake, Waldo Lake, Davis Lake and the Willamette Pass Ski Resort. LOCATION: We are located off Highway 58 (Willamette Pass) near Crescent Lake Junction, approximately 75 miles east of Eugene, 65 miles south of Bend, and 3 ½ hours south of Portland. We are situated in the Oregon Cascade Range adjacent to the Diamond Peak Wilderness Area and the Deschutes National Forest in one of Oregon's most beautiful areas. ELEVATION: Approximately 4600 feet, dry and healthy. TREES: Douglas Fir, Noble Fir, Cedar, Lodge pole and Ponderosa Pine. Ground cover is native bunch grass and deer forage, manzanita, wild strawberries and serviceberry. TOPOGRAPHY: Sloped to gently rolling with a spectacular view of surrounding peaks and wilderness area. SOIL: Sandy volcanic soil – there is no fill on any home site. RAIN & SNOW: Total approximately 160 inches per year, approximately 25" rain. Snow on ground November to March. Young and old enjoy the winter season with snowmobiles and cross-country skiing. RECREATIONAL Unlimited!! - Only minutes away, including: OPPORTUNITIES: Downhill skiing, cross-country skiing, snowmobiling, sledding, mountain biking, water-skiing, boating, fly fishing, lake fishing, bird watching, hiking, sailing, wind surfing, swimming & sunbathing on Crescent Lake Beaches, Horseback riding. LAKES & RIVERS: More than 80 lakes, rivers, streams and reservoirs within one hour. Odell Lake and Crescent Lake only 5 minutes away. FISHING: Brook Trout, Rainbow Trout, Dolly Varden, German Brown Trout, Mackinaws, Kokanee, Bass and Atlantic Landlocked Salmon are from 3 minutes to 1 hour. -

Geologic Framework of the Regional Ground-Water Flow System in the Upper Deschutes Basin, Oregon
Cover photographs: Top: Steelhead Falls on the Deschutes River near Crooked River Ranch, Oregon. Middle: Crooked River Canyon at Crooked River Ranch, Oregon. Bottom: North and Middle Sister with a wheel-line irrigation system in the foreground near Sisters, Oregon. (Photographs by Rodney R. Caldwell, U.S. Geological Survey.) U.S. Department of the Interior U.S. Geological Survey Geologic Framework of the Regional Ground-Water Flow System in the Upper Deschutes Basin, Oregon By KENNETH E. LITE JR. and MARSHALL W. GANNETT Water-Resources Investigations Report 02–4015 Prepared in cooperation with Oregon Water Resources Department; Cities of Bend, Redmond, and Sisters; Deschutes and Jefferson Counties; The Confederated Tribes of the Warm Springs Reservation of Oregon; and U.S. Environmental Protection Agency Portland, Oregon 2002 U. S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, Secretary U.S. GEOLOGICAL SURVEY CHARLES G. GROAT, Director The use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government. For additional information: Copies of this report may be purchased from: District Chief U.S. Geological Survey USGS Information Services 10615 S.E. Cherry Blossom Dr. Box 25286 Portland, OR 97216-3103 Denver, CO 80225-0286 E-mail: [email protected] Telephone: 1-888-ASK-USGS Internet: http://or.water.usgs.gov Suggested citation: Lite, K.E., Jr., and Gannett, M.W., 2002, Geologic framework of the regional ground-water flow system in the upper Deschutes Basin, Oregon: U.S. Geological Survey Water-Resources Investigations Report 02–4015, p. -

Pure Adventure Snowplay, Ski Towns and Trip Ideas Take Me to Timberline
2012-2013 TRIP PLANNER SKIOREGOSkiOregon.orgN PURE Adventure Snowplay, ski towns and trip ideas Take me to Timberline. Timberline Lodge was constructed and dedicated to the American people by President Franklin D. Roosevelt in during the throes of the Great Depression. It continues to operate today as a national historic landmark, ski area, hotel, and mountain retreat for all to enjoy. Visit and find the only trail-side lodging in Oregon, the longest snow season in the country, savory Northwest Alpine Fresh cuisine, and the best freestyle terrain parks on Mt. Hood! Lodge Reservations: Visit us on the web: -- TimberlineLodge.com AMERICA’S YEAR ’ROUND PLAYGROUND IN THE MT. HOOD NATIONAL FOREST SkiOregon2012 -2013 15 Snow Play Six more ways to enjoy the fluffy stuff, from snowshoeing to sled dog rides. By Kim Cooper Findling & Eileen Garvin 21 Pushing the Boundaries Get out of your comfort zone with cat skiing, night skiing, backcountry skiing and terrain parks. By Tina Lassen 23 Beyond the Slopes Après-ski: Places to relax after a day on the slopes. By Kimberly Gadette Mt. Hood Meadows Brian W. Robb Brian W. www.SkiOregon.org 3 Legendary terrain... Challenging, unique, compelling and fun. Enough variety and steeps to keep you intrigued. With six high speed quads and now RFID gates at every lift, we’re making it easier and faster to access your favorite terrain. No more waiting while others fumble for tickets. Get here and ride! • Direct to Lift – pay as you go pass • $79 Peak* Days (Save $10) (auto-charged to your credit card) • $59 Off-Peak (Save $15) • 1st and 5th day FREE! Then every 5th day Free! Contents Lodge at Suttle Lake, p. -
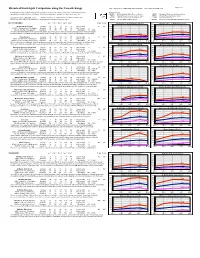
Historical Snowdepth Comparison Along the Cascade Range This Compilation Is ©2002-2005 Amar Andalkar
Page 1 of 5 Historical Snowdepth Comparison along the Cascade Range This compilation is ©2002-2005 Amar Andalkar www.skimountaineer.com Snowdepths in inches for the listed period of record, measured at snow courses, snow stakes, and automated gauges Data provided by: throughout the length of the Cascade Range. Note that snowfall data is available for only a few of these sites. BCRFC British Columbia River Forecast Centre NWAC Northwest Weather & Avalanche Center NRCS Natural Resources Conservation Service WRCC Western Regional Climate Center Updated through the 2003-2004 season. Italicized numbers are estimated based on limited available data. CCSS California Cooperative Snow Surveys NPS National Park Service See the end of the chart for an explanation of the Depth and Variability classification codes. WBSR Whistler Blackcomb Ski Resort ECNCA Environment Canada National Climate Archive BRITISH COLUMBIA Jan 1 Feb 1 Mar 1 Apr 1 May 1 Depth Variab 350 Bridge Glacier, N of Mt Meager (4600 ft) 350 Tenquille Lake, E of Mt Meager (5500 ft) Bridge Glacier (Lower) Average 47 61 65 69 61 Avg Snowfall — L LV 300 300 4600 ft (15 miles north of Mt Meager) Minimum 32 48 40 47 43 Max Snowfall — 250 250 BCRFC, monthly, 1995–present Maximum 63 88 117 108 87 Max Depth 117 (1999) 200 200 This fairly new measurement site is located at the eastern end of the Lillooet Icefield, in the next drainage north of the Mount Meager 171 150 150 Volcanic Complex. Snowdepths are relatively low but quite consistent due to its northerly location and icefield margin microclimate. -
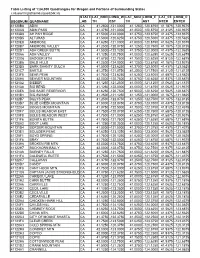
Table Listing of 1:24,000 Quadrangles for Oregon and Portions of Surrounding States C:Research\Gisthemes\Quad24k.Xls USGSNUM
Table Listing of 1:24,000 Quadrangles for Oregon and Portions of Surrounding States c:research\gisthemes\quad24k.xls STATE LAT_NOR LONG_W LAT_SOU LONG_E LAT_CE LONG_C USGSNUM QUADNAME _AB TH EST TH AST NTER ENTER 41120B8 ADIN CA 41.2500 -121.0000 41.1250 -120.8750 41.1875 -120.9375 41120C8 ADIN PASS CA 41.3750 -121.0000 41.2500 -120.8750 41.3125 -120.9375 41123D8 AH PAH RIDGE CA 41.5000 -124.0000 41.3750 -123.8750 41.4375 -123.9375 41120D5 ALTURAS CA 41.5000 -120.6250 41.3750 -120.5000 41.4375 -120.5625 41120E8 AMBROSE CA 41.6250 -121.0000 41.5000 -120.8750 41.5625 -120.9375 41120B7 AMBROSE VALLEY CA 41.2500 -120.8750 41.1250 -120.7500 41.1875 -120.8125 41122D1 ASH CREEK BUTTE CA 41.5000 -122.1250 41.3750 -122.0000 41.4375 -122.0625 41120A6 ASH VALLEY CA 41.1250 -120.7500 41.0000 -120.6250 41.0625 -120.6875 41122G6 BADGER MTN CA 41.8750 -122.7500 41.7500 -122.6250 41.8125 -122.6875 41123B8 BALD HILLS CA 41.2500 -124.0000 41.1250 -123.8750 41.1875 -123.9375 41123D5 BARK SHANTY GULCH CA 41.5000 -123.6250 41.3750 -123.5000 41.4375 -123.5625 41121C7 BARTLE CA 41.3750 -121.8750 41.2500 -121.7500 41.3125 -121.8125 41123F5 BEAR PEAK CA 41.7500 -123.6250 41.6250 -123.5000 41.6875 -123.5625 41120H6 BEAVER MOUNTAIN CA 42.0000 -120.7500 41.8750 -120.6250 41.9375 -120.6875 41121A2 BIEBER CA 41.1250 -121.2500 41.0000 -121.1250 41.0625 -121.1875 41121A8 BIG BEND CA 41.1250 -122.0000 41.0000 -121.8750 41.0625 -121.9375 41120E6 BIG SAGE RESERVOIR CA 41.6250 -120.7500 41.5000 -120.6250 41.5625 -120.6875 41121B1 BIG SWAMP CA 41.2500 -121.1250 41.1250 -121.0000 -

2018 Route Guide
2018 ROUTE GUIDE The Oregon Timber Trail is an iconic 670-mile backcountry mountain bike route spanning Oregon’s diverse landscapes from California to the Columbia River Gorge. © Dylan VanWeelden TABLE OF CONTENTS The small towns the route passes through, large amount of alpine singletrack and people of Oregon and Cascadia were truly special! - 2017 OTT Rider Overview . 6 By the numbers . 12 Logistics . 18 Getting There . 19 Stay Connected . 19 Is this route for you? . 19 Season & Climate . 20 Navigation and wayfi nding . 21 Resupply & water . 22 Camping and Lodging . 23 Leave No Trace . 24 Other trail users . 26 A note about trails . 27 4 © Gabriel Amadeus Disclaimer . 27 Springwater School Contribution . 27 Gateway Communities . 28 Fremont Tier . 34 Segment 1 of 10 - Basin Range . 40 Segment 2 of 10 - Winter Rim . 42 Segment 3 of 10 - Mazama Blowout . 46 Willamette Tier . 48 Segment 4 of 10 - Kalapuya Country . 52 Segment 5 of 10 - Bunchgrass Ridge . 56 Deschutes Tier . 60 Segment 6 of 10 - Cascade Peaks . 64 Segment 7 of 10 - Santiam Wagon Road . 67 Hood Tier . 70 Segment 8 of 10 - Old Cascade Crest. 75 Segment 9 of 10 - Wy’East. 78 Segment 10 of 10 - The Gorge. 80 Oregon Timber Trail Alliance . 84 5 Kim and Sam, Oregon Timber Trail Pioneers. © Leslie Kehmeier The Oregon Timber Trail is an iconic 670-mile backcountry mountain bike route spanning Oregon’s diverse landscapes from California to the Columbia River Gorge. The Oregon Timber Trail is a world-class bikepacking destination and North America’s premiere long- distance mountain bike route. -

Winter Trail Guide
SISTERS AREA CHAMBER OF COMMERCE SISTERS WINTERAREA CHAMBER OF COMMERCE TRAIL GUIDE Sisters Area Chamber of Commerce www.thesisterscountry.com Special thanks to EST SERVI FOR CE D E E P U S R A U R TMENT OF AGRICU L T DAY USE OF USFS TRAILS Always use good judgement when using or traveling over trails and roads. Some are not maintained and may be hazardous. Weather and other conditions can change without notice, so carry clothing for rain and cold temperatures. Always carry adequate water for all hikes and never drink trailside water from lakes and streams unless marked “potable” by the Forest Service. Food, matches, first-aid kit, flashlight, compass and maps are also essential. Deschutes and Willamette National Forest Maps, the McKenzie River National Recreation Trail Map , and the Three Sisters, Mt. Washington, and Mt. Jefferson Wilderness maps are available at Forest Service Stations. Mosquito repellent should also be carried along in late spring and summer months. As a safety precaution, always let someone know where you are going and when you expect to return. Dogs should be on a leash or controlled by voice command. Be sure to have appropriate parking and trail permits for specific destinations. The Sisters Area Chamber of Commerce and its members are not responsible for losses or injuries incurred when utilizing this information. Wilderness Areas and US Forest Service Land Uses Wilderness Areas have a delicate state of natural balance. Careless acts by people can upset this balance, resulting in destruction of the wilderness environment. The following practices will help preserve the wilderness for everyone’s enjoyment. -

Mt Bachelor Ski Area Improvements Final Environmental Impact Statement
FINAL ENVIRONMENTAL IMPACT STATEMENT: MT. BACHELOR SKI AREA IMPROVEMENTS PROJECT Prepared by: Bend-Ft. Rock Ranger District Deschutes National Forest Bend, OR With the assistance of: Cirrus Ecological Solutions, LC Logan, UT February 2013 The United States Department of Agriculture (USDA) prohibits discrimination in its programs on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, and marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotapes, etc.) should contact the USDA Office of Communications at (202) 720-2791. To file a complaint, write the Secretary of Agriculture, U.S. Department of Agriculture, Washington, D.C. 20250 or call 1-800-245-6340 (voice) or (202) 720-1127 (TDD). USDA is an equal opportunity employer. Final Environmental Impact Statement: Mt. Bachelor Ski Area Improvements Project Deschutes County, Oregon Lead Agency: USDA Forest Service Responsible Official: John Allen, Forest Supervisor Deschutes National Forest 63095 Deschutes Market Rd. Bend, OR 97701 For Information Contact: Amy Tinderholt Recreation, Special Uses and Trails Team Leader Bend / Fort Rock Ranger District Ranger (541) 383-4708 Abstract: The Bend-Ft. Rock Ranger District, Deschutes National Forest (DNF) has received a proposal from Mt. Bachelor, Inc., operators of Mt. Bachelor ski resort, requesting authorization to implement improvements included in their recently revised master development plan (MDP). The MDP documents analysis of current conditions at the resort and, based on that analysis, outlines anticipated development and management of the resort over the next 10 years. -

West Bend Trails Map (PDF)
NW en To Sh ok p Trail ev Br lin P Shevlin Park ark R oad NW Newpor !B t Ave S . 2 o 0 9 8 d Broken Top 7 3 46 !B 3 9 01 a # C 38 M razek R 0 r T 460 NW Galveston Ave . T ra 1 T o il r dig 06 t a n 4601 S il i 46 Road Bend -W iners M h l t l T s ky i 370/380 Road !B iu 46 S un 4 a o l o t W N tor 1 r o o il l lands Connec a d t rth k Tra i ade High T e F M ra ze a sc s d o r W r M r zek T Ca h k a N 3 M k i T 7 T F r n to g da Cre e r 8 r ec So a # a n t r Phil's n o il e w T C reek Pl o 2 u o 2 T l ay C n 4 e m al r a i Are s 0 le Fo rk . l a d S d S 2 l D 7 Br id w U n k k id a T ek !B la ee 3 g M p r re 4 h y r e m a C g r C h alo i li i i 01 m H n i a l S C p 46 Tu sca de v d ll ky ly a e e o r y O n C S e il n li 47 R ek T ra L l n l O y i l 1 a a e y il r a U nl n F k 400 ph ill o h s R 16 r n c l e o Three ad T h a 4603 ail ly w g s Tr n o 4 r e 0 M R ine nly O i B D 6 l T l l O Tumalo k y il l 4 o h T i o Cree k p il T Sisters l S a n r a U 17 h eek T l a e r m d a C rail Tu 0 i p B 6 B Falls M e !B 1 ra U l r v Todd 0 e il g 3 i v in 0 i r id T G a 3 s G 1 7 i l Br rail l r r r T i Wilderness d i alo reek !B s a 0 Lake 3 ra Tum C hoo p K T D T g T W 36 r a e ek 4 e Cre s d l r Closed to Mechanized use reek Skyliners ai 24 t' 48 ry v r r n e i e C il T T e tu R 6 K n a g S a y 0 n i id r nl ps T 0 l 7 e s r 0 14 i (NO BIKES) l w T O X 5 C B e hill o 3 T rk g n o E 1 a e o id Tr l l ow 3 K r t F ai r W Todd Lake a h R i D h il T 49 The S t a 0 a !B m o u u City of Bend S r Up W e a lo hi 25 i Tetherow T l l n r KG B l Lair h p