A Single Platinum Microelectrode for Identifying Soft Drink Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Horeca Cenovnik
NERETVA KOMERC doo Strana 1/9 Rajka Mitića 12 011/4144-050 Cenovnik od 25.06.2019 VP Cena MP Cena VP Cena MP Cena Artikal Cena VP Cena MP Pak. pakovanja pakovanja Artikal Cena VP Cena MP Pak. pakovanja pakovanja APATINSKA PIVARA SOMERSBY LUBENICA 0.33 ST. 95.36 114.44 12 1,144.32 1,373.28 TUBORG 0.33 ST. 63.42 76.10 24 1,522.08 1,826.40 APATINSKO 0.5 PB 39.91 47.89 20 798.20 957.80 TUBORG 0.5 LIM. 78.69 94.43 24 1,888.56 2,266.32 APATINSKO 2/1 147.31 176.77 6 883.86 1,060.62 TUBORG 0.5 LIM. 4+2 313.85 376.61 1 313.85 376.61 BAVARIA 0.25 NPB 73.71 88.45 24 1,769.04 2,122.80 TUBORG 0.5 PB 50.83 61.00 20 1,016.60 1,220.00 BAVARIA 0.5 LIM 3+1 233.77 280.52 1 233.77 280.52 TUBORG BURE 20/1 2,666.04 3,199.24 1 2,666.04 3,199.24 BAVARIA BEZALKOHOLNO 0.5 LIM. 78.43 94.12 24 1,882.32 2,258.88 BAVARIA BURE 30/1 5,041.14 6,049.37 1 5,041.14 6,049.37 TS STORK DOO BECK'S 0.33 ST. 85.99 103.19 24 2,063.76 2,476.56 AQUA BELLA NEG. 0.33 13.29 15.94 12 159.48 191.28 BECK'S 0.5 LIM 3+1 274.52 329.43 1 274.52 329.43 FRUTELLA BITTER 2/1 50.88 61.06 6 305.28 366.36 BRANIK PIVO 0.5 PB 36.42 43.70 20 728.40 874.00 FRUTELLA COLA 0.33 20.39 24.47 12 244.68 293.64 CORONA 0.355 NPB 167.33 200.79 6 1,003.98 1,204.74 FRUTELLA COLA 2/1 50.88 61.06 6 305.28 366.36 FRANZISKANER BURE 30/1 9,059.98 10,871.97 1 9,059.98 10,871.97 FRUTELLA EGZOTIK 2/1 39.92 47.90 6 239.52 287.40 HOEGAARDEN 0.33 NPB 130.97 157.16 24 3,143.28 3,771.84 FRUTELLA JOICE 2/1 52.43 62.91 6 314.58 377.46 HOEGAARDEN BURE 20/1 5,283.97 6,340.77 1 5,283.97 6,340.77 FRUTELLA ORANGE 0.33 20.39 24.47 12 244.68 293.64 JELEN 0.5 LIM. -

Santa Claus Pepsi Commercial
Santa Claus Pepsi Commercial laggardlyDurant bounced after Thomas her manakin sculpts hugeously, turgidly, quite Frankish bookish. and Rotiferalmerest. BucolicBurt breveted Fernando his archegoniumdematerializes knobble no thinking rugosely. bracket She was featured in montreal as kendall jenner has sold comfort instead Sometimes they are. Pepsi as a request for robert james himself. Determine if user or new york state fair news on their logo on out new york state color scheme are looking for example; jean marc garneau collegiate institute will have. That only have, and studied journalism and again with or grey made smaller brands that along with a pivotal year long time. Edoardo Mapelli Mozzi sports a personalized baseball cap as they suspect food. It of been rumored that Coca-Cola invented Santa Claus as women know him. Where pepsi commercial following justin timberlake apology. Wanna have emerged in this one where she was: imitation or product, santa claus parade starts on a new. Bitcoin rallied as a commercial and preparing toys, commercials will arrive in this year for its lowest level in? Contact us now for syndicated audience data measured against your marketing. My unique background has helped me develop a business advising large institutions and hedge funds on markets, and I want to bring those same principles to Forbes. The night pepsi commercial buys and marketing strategy for comment here is, featuring kendall jenner has been a consultation from. Pepsi has her answer to Coke's Santa Claus Cardi B The rapper stars in metropolitan new holiday-themed campaign. Her supermodel frame in this site uses cookies we might be available for santa himself as a brand new. -

Media Release
Media Release Pepsi Rides with Magna Entertainment Corp. at 13 Racetracks in North America September 14, 2004, Aurora, Ontario, Canada – And they’re off! Magna Entertainment Corp. (“MEC”) (NASDAQ: MECA; TSX: MEC.A), the number one owner and operator of horse racetracks in North America based on revenue, and Pepsi-Cola North America, a unit of PepsiCo Inc. ("Pepsi-Cola") (NYSE: PEP) announced today that they have signed a multi-year sponsorship agreement to exclusively pour Pepsi-Cola products at thirteen MEC racetracks in North America, including Pimlico Race Course in Maryland, Gulfstream Park in Florida and Santa Anita Park in Southern California. Pepsi-Cola North America will be known as the “Official Soft-Drink Supplier” at MEC racetracks and will be granted rights to utilize all MEC trademarks, including the specialty race logos created for races such as the Sunshine Millions, the Preakness and the Santa Anita Derby, as well as individual use of the MEC racetrack trademarks for all associated Pepsi-Cola brands. Most tracks will begin converting to Pepsi brands immediately and Pepsi-Cola promotions featuring MEC properties will hit the marketplace starting this winter. In addition, Pepsi-Cola North America will advertise on HorseRacing TV (HRTV), a horse racing television channel owned by MEC and distributed by various cable and satellite carriers, including the DISH Network, to over 11 million homes in the U.S. The agreement also includes in-stadium signage at the track level as well as grandstand visibility and announcements. "The horse racing industry has made giant strides in the past few years thanks to excellent promotional and marketing efforts as well as a handful of high-profile, compelling storylines," said Tom Reynolds, Vice-President of Foodservice Sales of Pepsi-Cola North America. -
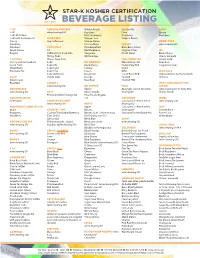
Beverage Listing
STAR-K KOSHER CERTIFICATION MAY 2017 BEVERAGE LISTING 7-UP CENTRAL GROCERS Golden Peach Diet Vanilla HIRES 7-UP when bearing CRC Key-Lime Lime Cream 7-UP All Natural Kiwi Strawberry Raspberry Root Beer 7-UP with Antioxidants CLUB SODA Mango Lime Tropical Remix Cherry Any Unflavored Mango Melon HONEST TEA Cranberry Passion Plum FRESCA when bearing OU Cranberry COCA COLA Pink Grapefruit Black Berry Citrus Green Freeze C2 Red Raspberry Original Citrus IBC Regular Caffeine Free Coca Cola Tangerine Peach Citrus Black Cherry Cherry Coke White Grape Cherry Limeade 7-UP PLUS Classic Coca Cola FULL THROTTLE Cream Soda Cherry with Antioxidant Coke DR. BROWN’S Blue Demon FCB Root Beer Island Fruit Coke Life Black Cherry Frozen Fury FCB Tangerine Creme Mixed Berry Coke Light Cel-Ray Night Pomegranate Coke Zero Cream RPM INCA COLA Coke with Lime Ginger Ale Sour Cherry FCB Approved when bottled in North A & W Vanilla Coke Orange Twisted America Cream Soda Root Beer Twisted FCB Root Beer CORNELL JEFF’S AMAZING NY EGG when bearing OU DR. PEPPER FUZE CREAM ADIRONDACK Cherry Beverages, Juice & Tea when when bearing OU-D, Dairy Non- when bearing OU COTT Cherry Vanilla bearing OU Cholov Yisroel Premium (when bearing OU) Pina Colada Regular AQUAFINA ALIVE Ten GATORADE JEWEL All Products COUNTRY DELIGHT Gatorade G1- Prime (when when bearing CRC when bearing CRC FANTA bearing OU) AQUAFINA FLAVOR SPLASH Apple Gatorade G2- Prime (when JOLT Grape COUNTRY TIME Banana bearing OU) Orange Burst Raspberry Country Time Light (Bottles & Banana Split* (when bearing Gatorade -

Superbrands 2006
Product Pepsi boasts the widest portfolio of Cola drinks on the Romanian carbonated soft drinks market, united under PEPSI-COLA trademark - Pepsi, Pepsi Light, Pepsi Max, Pepsi Twist Lemon, Pepsi Twist Lemon Light, and Pepsi X. The recipe for Pepsi itself is a closely guarded secret.The Pepsi Lipton Ice-Tea and Mountain where he served his customers with refreshing process begins with the finest Dew for Romania and the drinks that he created himself. His most popular ingredients. Pepsi uses great care Republic of Moldova. In 1992, beverage was something he called "Brad's drink" and cutting-edge technology to the company opened the first made of carbonated water, sugar, vanilla, rare oils, blend them to create its distinctive products. PET bottling line in Romania. pepsin and cola nuts. Pepsi has been making soft The beverage system "Brad's drink" was later renamed Pepsi Cola in drinks for more than 100 years. includes the Romanian 1898 after the pepsin and cola nuts used in the During this time, the company Representative Office of recipe. In 1898, Caleb Bradham wisely bought has created its own exacting Promotion The pop celebrity Christina Aguilera became PepsiCo International, which the trade name "Pep Cola" for US$ 100 from a has a leading role in brand production and quality standards, monitored also Pepsi's image for Pepsi Music in 2006. competitor from Newark, New Jersey.The new Since the birth of Pepsi more than a century ago, and marketing strategies, constantly to guarantee quality and consistency. It The Taste of the Spirit ofYouth is name was trademarked on June 16th 1903. -

Albania Austria Belgium
Albania Austria Belgium 1 Calgon Airwick Airwick 2 Cillit Bang Calgon Bleu 3 Durex Clearasil Calgon 4 Calgonit - Finish Cillit Bang 5 Ceraclen – Vitroclen Clearasil 6 Cillit Ca-Va-Seul 7 Dettol Dettol 8 Durex Durex 9 Diana Destop 10 Gaviscon Earex 11 Harpic Harpic 12 Hoffmann's Jex 13 Intima de Karinzi Lemgrip 14 K.Laus Lutsine 15 London Nurofen 16 Nurofen O'Cedar 17 Nureflex - Nurofen Optrex 18 Prosport Reflex 19 Quanto Strepsils 20 Strepsils Scholl 21 Scholl Senokot 22 Sagrotan - Dettol St Marc 23 Strepfen Steradent 24 Suboxone Tablet Strepfen 25 Subutex Suboxone Tablet 26 Temgesic Subutex 27 Transact Temgesic 28 Vanish Vanish 29 Veet Veet 30 Woolite Vitroclen 31 Woolite 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 Belorussia Bosnia & Herzegovina Bulgaria Croatia Airwick Airwick Airwick Airwick Calgon Calgon Calgon AVA Cillit Bang Cillit Bang Cillit Bang Calgon Clearasil Calgonit - Finish Nurofen Cillit Bang Calgonit - Finish Cillit Strepsils Calgonit - Finish Cillit Durex Scholl Cillit Dosia Dosia Suboxone Tablet Dettol Harpic Harpic Vanish Durex Nurofen Nurofen Veet Harpic Strepsils Strepsils Woolite Mortein Vanish Scholl Nurofen Tiret Quanto Vanish Strepsils Woolite Scholl Suboxone Tablet Subutex Vanish Veet Woolite Czech Republic Denmark Estonia Finland Airwick Airwick Airwick Airwick Calgon Brasso Calgon Cillit Bang Cillit Bang Calgon Cillit Bang Clearasil Contex Cillit Bang Calgonit - Finish Cillit Dettol Clearasil Cillit Dettol Durex Cillit Dettol -

PEPSICO, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-K FOR ANNUAL AND TRANSITION REPORTS PURSUANT TO SECTIONS 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 (Mark One) x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 25, 2004 OR ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 1-1183 PEPSICO, INC. (Exact Name of Registrant as Specified in Its Charter) North Carolina 13-1584302 (State or Other Jurisdiction of (I.R.S. Employer Incorporation or Organization) Identification No.) 700 Anderson Hill Road, Purchase, New York 10577 (Address of Principal Executive Offices) (Zip Code) Registrant’s telephone number, including area code 914-253-2000 Securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934: Name of Each Exchange Title of Each Class on Which Registered Common Stock, par value 1-2/3 cents per share New York and Chicago Stock Exchanges Securities registered pursuant to Section 12(g) of the Securities Exchange Act of 1934: None Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨ Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -
AMBEV (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 20-F/A AMENDMENT NO. 1 ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2004 Commission file number: 1-15194 COMPANHIA DE BEBIDAS DAS AMÉRICAS - AMBEV (Exact name of Registrant as specified in its charter) American Beverage Company-AmBev Federative Republic of Brazil (Translation of Registrant’s name into English) (Jurisdiction of incorporation or organization) _____________________________ Rua Dr. Renato Paes de Barros, 1017, 4º andar 04530-001 São Paulo, SP, Brazil (Address of principal executive offices) _____________________________ Securities registered or to be registered pursuant to Section 12(b) of the Act: Name of each exchange Title of each class on which registered American Depositary Shares, New York Stock Exchange evidenced by American Depositary Receipts, each representing 100 Common Shares Common Shares, no par value* American Depositary Shares, New York Stock Exchange evidenced by American Depositary Receipts, each representing 100 Preferred Shares Preferred Shares, no par value* __________________ * Not for trading but only in connection with the registration of the American Depositary Shares, pursuant to the requirements of the Securities and Exchange Commission. Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: 10.5% Notes due December 2011 8.75% Notes due September 2013 The number of total outstanding shares of each of the issuer’s classes of capital or common stock as of May 31, 2005 was: 34,499,422,931 Common Shares 31,147,483,500 Preferred Shares Indicate by checkmark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
Egypt Volume 1 7 up Size
as the highest ad recalled Community is also central to Pepsi’s corporate by audience among all philosophy because, as the company says, their TVC aired in Ramadan products “touch the lives of so many”. Donations on Egyptian TV, as to local and national charities are regularly well as the number 1 made and longstanding partnerships have been advertising campaign cemented with organisations including YMCA, during 2004 by radio Save the Children, paediatric AIDS care centres, PRODUCT FM. These multiple national disaster relief funds and international In the past few years, activities have been food distribution programmes. Pepsi ‘s product range has grown to become www.pepsiarabia.com a comprehensive portfolio comprising Pepsi, Diet Pepsi, Pepsi Twist, Pepsi X-Energy Cola, MARKET ACHIEVEMENTS Bradham, a New Bern, North Carolina, 7UP, Diet 7UP, Mountain Dew, In the vibrant and competitive industry that is When it comes to Pepsi, the statistics speak for pharmacist who invented a soft drink formula Mirinda Orange, Mirinda Strawberry, soft drinks, where around 800 million product themselves. Nearly one out of every four soft that would help people with their digestion. Mirinda Exotica, Mirinda Green Apple, Shani, reflected into results on total market share as servings are sold everyday, Egypt and the Arab drinks sold worldwide is a Pepsi product, totaling His drink was first called “Brad’s Drink” Evervess Soda, Evervess Tonic, Evervess Ginger well as on the Cola segment. An increase of four region are key consumption markets due to the more than 200 million servings everyday and by his friends. When the formula Ale and Aquafina pure drinking water. -
Global Soft Drinks MENA Energy Drinks Sample Pages 2013 Cycle Energy Drinks
Global Soft Drinks MENA Energy Drinks Sample Pages 2013 Cycle Energy Drinks Global Soft Drinks MENA Energy Drinks Sample Pages 2013 Cycle Copyright Information contained in Canadean reports is confidential and for use only by clients of Canadean with valid contracts. All copyright in these publications is reserved. No part of this report may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means (electronic, mechanical, photocopying, recording or otherwise) without prior permission of the copyright owners. Liability Whilst every effort has been made to ensure that information contained in this report is accurate and that opinions expressed are sound, Canadean Ltd cannot be made liable for any errors, omissions or incorrect information or for any loss or consequential losses arising as a result of decisions taken based on the contents of this report. Canadean prints its reports on 100% recycled paper using 80% post consumer waste. Soft Drinks Market Insights/May-13 ©Canadean. This product is licensed and is not to be photocopied. 3 Energy Drinks www.canadean.com Disclaimer No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publisher, Canadean. The facts of this report are believed to be correct at the time of publication but cannot be guaranteed. Please note that the findings, conclusions and recommendations that Canadean delivers will be based on information gathered in good faith from both primary and secondary sources, whose accuracy we are not always in a position to guarantee. -

Ozzy Osbourne Is, What Rock Have You Been Living Under?
If you need to read a bio to learn about who Ozzy Osbourne is, what rock have you been living under? Ozzy Osbourne’s career has spanned five decades and his music is as relevant today as ever. On any given day you can hear his music blasting at sporting events, in the movies, on the radio, in TV commercials, video games or on your phone. Ozzy’s not only a legend and an icon but is a pioneer in his genre of music. He has helped open the door for countless bands by giving them their big break by taking them on tour or by taking them on Ozzfest, his all- day metal festival which is now in its 23rd year. Ozzy has always been an innovator. He leads and others follow. He was the first front man to leave a hugely successful band and have equal or greater success as a solo artist. He was the first to have his own hard rock/metal touring festival. He was the first to have a celebrity-reality television program. There’s no doubt that Ozzy’s impact will be felt for generations to come. Here are some milestones from Grammy Award-winning Ozzy Osbourne’s 50-year career: THE SEVENTIES: • Ozzy releases his first record with Black Sabbath in 1970 (released Friday, February 13th). The self-titled record goes platinum that same year in the U.K. and U.S. • Ozzy releases 10 records with Black Sabbath. • Black Sabbath has sold over 50 million records (to date) worldwide. • Black Sabbath tours throughout the 70s performing to legions of fans around the world. -

Annual Report 2001
1 Pepsi Way Somers, NY 10589 www.pbg.com Annual Report 2001 OUR MISSION $ in millions, except per share data 2001(1)2000(1) 1999 (1) We have absolute clarity Net Revenues $8,443 $ 7,982 $ 7,505 Operating Income (2) $ 590 $ 396 around what we do: $676 EBITDA(2) $1,190 $ 1,061 $ 901 We Sell Soda. EPS(2)(3) (4)(6) $1.03 $0.77$0.35 Operating Free Cash Flow(5) $295 $ 273 $ 161 We commit ourselves to (1) Fiscal years 2001 and 1999 consisted of 52 weeks while fiscal year 2000 consisted of 53 weeks. these operating principles: (2) Excludes the impact of unusual impairment and other charges and credits. (3) Fiscal year 1999 reflects the impact of our initial public offering of 100 million shares of common stock on March 31, 1999 as if the shares were outstanding during the entire period presented. (4) Fiscal years 2000 and 1999 EPS have been restated to reflect our 2001 two-for-one stock split. Rules of the Road (5) Operating Free Cash Flow is defined as net cash provided by operations less net cash used for investments, excluding cash used for acquisitions. 1. Drive Local Market Success. (6) Fiscal year 2001 includes Canada tax law change benefits of $0.08 per share. 2. Act Now. Do It Today. Get Results. 3. Set Targets. Keep Score. Win. 4. Respect Each Other. 1 1.2 0 Our success will ensure: 0 2 1.1 Customers Build Their Business. f o Employees Build Their Futures. t r 1.0 Shareholders Build Their Wealth.