Factors Influencing Freshwater Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surface Water Ambient Network (Water Quality) 2020-21
Surface Water Ambient Network (Water Quality) 2020-21 July 2020 This publication has been compiled by Natural Resources Divisional Support, Department of Natural Resources, Mines and Energy. © State of Queensland, 2020 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Summary This document lists the stream gauging stations which make up the Department of Natural Resources, Mines and Energy (DNRME) surface water quality monitoring network. Data collected under this network are published on DNRME’s Water Monitoring Information Data Portal. The water quality data collected includes both logged time-series and manual water samples taken for later laboratory analysis. Other data types are also collected at stream gauging stations, including rainfall and stream height. Further information is available on the Water Monitoring Information Data Portal under each station listing. -
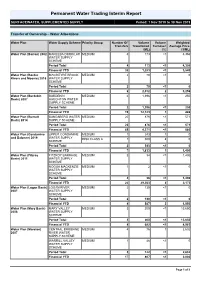
Permanent Water Trading Interim Report
Permanent Water Trading Interim Report SURFACEWATER, SUPPLEMENTED SUPPLY Period: 1 Nov 2019 to 30 Nov 2019 Transfer of Ownership – Water Allocations Water Plan Water Supply Scheme Priority Group Number Of Volume Volume Weighted Transfers Transferred Turnover Average Price (ML) (%) ($/ML) Water Plan (Barron) 2002 MAREEBA DIMBULAH MEDIUM 4 173 <1 4,356 WATER SUPPLY SCHEME Period Total 4 173 <1 4,356 Financial YTD 35 1,283 <1 3,540 Water Plan (Border MACINTYRE BROOK MEDIUM 2 70 <1 0 Rivers and Moonie) 2019 WATER SUPPLY SCHEME Period Total 2 70 <1 0 Financial YTD 6 2,012 2 3,259 Water Plan (Burdekin BURDEKIN MEDIUM 2 1,396 <1 250 Basin) 2007 HAUGHTON WATER SUPPLY SCHEME Period Total 2 1,396 <1 250 Financial YTD 19 12,123 1 486 Water Plan (Burnett BUNDABERG WATER MEDIUM 20 876 <1 571 Basin) 2014 SUPPLY SCHEME Period Total 20 876 <1 571 Financial YTD 69 4,271 <1 580 Water Plan (Condamine UPPER CONDAMINE MEDIUM 1 243 1 0 and Balonne) 2019 WATER SUPPLY RISK CLASS A 1 300 4 0 SCHEME Period Total 2 543 <1 0 Financial YTD 7 1,833 1 1,400 Water Plan (Fitzroy FITZROY BARRAGE MEDIUM 3 34 <1 1,400 Basin) 2011 WATER SUPPLY SCHEME NOGOA MACKENZIE MEDIUM 1 2 <1 0 WATER SUPPLY SCHEME Period Total 4 36 <1 1,400 Financial YTD 22 29,563 8 2,173 Water Plan (Logan Basin) LOGAN RIVER MEDIUM 2 130 <1 0 2007 WATER SUPPLY SCHEME Period Total 2 130 <1 0 Financial YTD 4 587 3 1,050 Water Plan (Mary Basin) MARY VALLEY MEDIUM 3 200 <1 13,650 2006 WATER SUPPLY SCHEME Period Total 3 200 <1 13,650 Financial YTD 8 682 <1 4,983 Water Plan (Moreton) CENTRAL BRISBANE MEDIUM -

Logan River Vision
LOGAN RIVER VISION RIVER HEALTH RIVER DESTINATIONS RIVER PLAY Logan City Council acknowledges the Traditional Custodians of the land, pays respect to Elders past and present, and extends that respect to all Aboriginal and Torres Strait Islander peoples in the City of Logan. VISION STATEMENT In 2067, the Logan River is a world class environmental asset that is accessible to everyone, is celebrated and will connect people and places along its length from the mountains to the bay. LOGAN RIVER VISION OUTCOMES COMMUNITY ENGAGEMENT The Logan River will continue to provide In 2016, over a 10 week period we heard LOGAN RIVER VISION many benefits for residents and visitors. from hundreds of residents across the It’s a place of spiritual significance and a city about what they want the Logan natural resource for drinking and irrigation, River to be like in 50 years’ time. From areas for leisure and recreational activities this, we received 678 ideas and engaged as well as a key wildlife corridor from the with approximately 10,000 community mountains to the bay. members both online as well as through The Logan River Vision is a 50 year over 14 community activities across vision from 2017 through to 2067. It was the City of Logan. The content of these developed from ideas and feedback from pages of the Logan River Vision has been the community and will: compiled from information received from the community of Logan. The concept + support a healthy and clean river sketches included in these pages are + allow for continued urban and provided as examples of how a range population growth of themes can be applied to achieve the vision for the Logan River. -

Surface Water Network Review Final Report
Surface Water Network Review Final Report 16 July 2018 This publication has been compiled by Operations Support - Water, Department of Natural Resources, Mines and Energy. © State of Queensland, 2018 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Interpreter statement: The Queensland Government is committed to providing accessible services to Queenslanders from all culturally and linguistically diverse backgrounds. If you have difficulty in understanding this document, you can contact us within Australia on 13QGOV (13 74 68) and we will arrange an interpreter to effectively communicate the report to you. Surface -

Baddiley Peter Second Statement Annex PB2-816.Pdf
In the matter of the Commissions of Inquiry Act 1950 Commissions of Inquiry Order (No.1) 2011 Queensland Floods Commission of Inquiry Second Witness Statement of Peter Baddiley Annexure “PB2-8(16)” PB2-8(16) 1 PB2-8(16) 2 PB2-8 (16) FLDWARN Coastal Rs Maryborough south 1 December 2010 to 31 January 2011 TO::BOM612+BOM613+BOM614+BOM615+BOM617+BOM618 IDQ20780 Australian Government Bureau of Meteorology Queensland FLOOD WARNING FOR COASTAL STREAMS AND ADJACENT INLAND CATCHMENTS FROM MARYBOROUGH TO THE NSW BORDER Issued at 6:46 PM on Saturday the 11th of December 2010 by the Bureau of Meteorology, Brisbane. Heavy rainfall during Saturday has resulted in fast level rises in coastal catchments and adjacent inland catchments. The heaviest rainfall to 6pm Saturday has been in the Pine Rivers area and coastal areas from Brisbane to the Gold Coast. Further rainfall is forecast overnight with fast rises and some minor flooding expected. Rainfall totals in the 9 hours to 6pm include: Wynnum 100mm, Mitchelton 76mm, Logan 65mm, Coomera 46mm , Brisbane 74mm and Beerwah 60m. ## Next Issue: The next warning will be issued by 8am Sunday. Latest River Heights: nil. Warnings and River Height Bulletins are available at http://www.bom.gov.au/qld/flood/ . Flood Warnings are also available on telephone 1300 659 219 at a low call cost of 27.5 cents, more from mobile, public and satellite phones. TO::BOM612+BOM613+BOM614+BOM615+BOM617+BOM618 IDQ20780 Australian Government Bureau of Meteorology Queensland FLOOD WARNING FOR COASTAL STREAMS AND ADJACENT INLAND CATCHMENTS FROM MARYBOROUGH TO BRISBANE Issued at 8:19 AM on Sunday the 12th of December 2010 by the Bureau of Meteorology, Brisbane. -

15. Members of Tne Nerang Kiver Tribe at Their Campsite Near Southport
15. Members of tne Nerang Kiver tribe at their campsite near Southport, circa 1889. 16. THE GOLD COAST: ITS FIRST INHABITANTS by R.I. Longhurst B.A. (Hons) A.L.A.A.* We know very little about the aboriginal inhabitants of the Gold Coast, or at least much less than we know about neighbouring areas where tribal members survived to be interviewed by scientific researchers this century. Much of what we do know comes from the memoirs of early European settlers who more often than not regarded the few surviving aborigines as pitiful novelties, the objects of charity in the form of ragged clothing and alcohol. Only a very few, such as the early timber-getter Edmund Harper, ever tried to understand and learn from them. Certainly, by 1900 there remained only a few fiill-blooded natives of the original South Coast tribes. The 1901 Queensland census was the first to actually provide statistics of the aboriginal population of the state. In the Logan stat istical division of which the Gold Coast was only a very small part, eighty-one aborigines, both full- and half-blood, were counted. This figure comprised forty-nine males, and thirty- two females.1 This compares with an estimate of 1500 to 2000 natives living in the watershed of the Logan, Albert, Coomera and Nerang Rivers in the 1850s.2 Harper, reminiscing in 1894, recalled the 'Tulgigin' tribe of the North Arm of the Tweed, numbering 'some two hundred. The men were nearly all big stout fellows, some of them over six feet in height, and weighing up to fourteen stone'.3 Laila Haglvmd's archaeological -

South-East Queensland Water Supply Strategy Environmental
South-east Queensland Water Supply Strategy Environmental Assessment of Logan/Albert and Mary Catchment Development Scenarios FINAL DRAFT Study Team Dr Sandra Brizga, S. Brizga & Associates Pty Ltd (Study Coordinator) Professor Angela Arthington, Griffith University Mr Pat Condina, Pat Condina and Associates Ms Marilyn Connell, Tiaro Plants Associate Professor Rod Connolly, Griffith University Mr Neil Craigie, Neil M. Craigie Pty Ltd Dr Mark Kennard, Griffith University Mr Robert Kenyon, CSIRO Mr Stephen Mackay, Griffith University Mr Robert McCosker, Landmax Pty Ltd Ms Vivienne McNeil, Department of Natural Resources, Mines & Water Logan/Albert and Mary Catchment Scenarios Environmental Assessments Table of Contents Table of Contents...........................................................................................................2 List of Figures................................................................................................................3 List of Tables .................................................................................................................3 Executive Summary.......................................................................................................6 Scope and Objectives.................................................................................................6 General Overview of Key Issues and Mitigation Options.........................................6 Logan/Albert Catchment Development Scenarios.....................................................7 Mary Catchment -

Information Needs for Freshwater Flows Into Estuaries
Information needs for freshwater flows into estuaries 1 Information needs for freshwater flows into estuaries Assessment of Information Needs for Freshwater Flows into Australian Estuaries Final Report April 2006 Scheltinga, D.M., Fearon, R., Bell, A. and Heydon, L. FARI Australia Pty Ltd and the Cooperative Research Centre for Coastal Zone, Estuary and Waterway Management. This document was commissioned by the Fisheries Research and Development Corporation (FRDC) and Land and Water Australia (LWA). For copies please contact: Crispian Ashby Richard Davis Programs Manager Coordinator, Environmental Water Fisheries Research and Development Allocation Program Corporation Land and Water Australia PO Box 222 Canberra DEAKIN WEST ACT 2600 Email: [email protected] Email: [email protected] www.frdc.com.au OR Coastal CRC 80 Meiers Rd Indooroopilly Qld 4068 Ph: 07 3362 9399 www.coastal.crc.org.au While every care is taken to ensure the accuracy of this publication, FARI Australia Pty Ltd and the Cooperative Research Centre for Coastal Zone, Estuary and Waterway Management disclaims all responsibility and all liability (including without limitation, liability in negligence) and costs which might incur as a result of the materials in this publication being inaccurate or incomplete in any way and for any reason. 2 Information needs for freshwater flows into estuaries Table of contents 1 Introduction ................................................................................................................ 5 2 The Value of Australia’s -

Timeline for Brisbane River Timeline Is a Summary of Literature Reviewed and Is Not Intended to Be Comprehensive
Assessing Historical Change in Coastal Environments Port Curtis, Fitzroy River Estuary and Moreton Bay Regions NC Duke P Lawn CM Roelfsema KN Zahmel D Pedersen C Harris N Steggles C Tack Historical Coastlines HISTORICAL COASTLINES Assessing Historical Change in Coastal Environments Port Curtis, Fitzroy River Estuary and Moreton Bay Regions Norman C. Duke, Pippi T. Lawn, Chris M. Roelfsema, Katherine N. Zahmel, Dan K. Pedersen, Claire Harris, Nicki Steggles, and Charlene Tack Marine Botany Group Centre for Marine Studies The University of Queensland Report to the CRC for Coastal Zone Estuary and Waterway Management July 2003 HISTORICAL COASTLINES Submitted: July 2003 Contact details: Dr Norman C Duke Marine Botany Group, Centre for Marine Studies The University of Queensland, Brisbane QLD 4072 Telephone: (07) 3365 2729 Fax: (07) 3365 7321 Email: [email protected] Citation Reference: Duke, N. C., Lawn, P. T., Roelfsema, C. M., Zahmel, K. N., Pedersen, D. K., Harris, C. Steggles, N. and Tack, C. (2003). Assessing Historical Change in Coastal Environments. Port Curtis, Fitzroy River Estuary and Moreton Bay Regions. Report to the CRC for Coastal Zone Estuary and Waterway Management. July 2003. Marine Botany Group, Centre for Marine Studies, University of Queensland, Brisbane. COVER PAGE FIGURE: One of the challenges inherent in historical assessments of landscape change involves linking remote sensing technologies from different eras. Past and recent state-of-the-art spatial images are represented by the Queensland portion of the first map of Australia by Matthew Flinders (1803) overlaying a modern Landsat TM image (2000). Design: Diana Kleine and Norm Duke, Marine Botany Group. -

Baddiley Peter Second Statement Annex PB2-831.Pdf
In the matter of the Commissions of Inquiry Act 1950 Commissions of Inquiry Order (No.1) 2011 Queensland Floods Commission of Inquiry Second Witness Statement of Peter Baddiley Annexure “PB2-8(31)” PB2-8(31) 1 PB2-8(31) 2 PB2-8 (31) Queensland Flood Warning Summary 1 December 2010 to 31 January 2011 IDQ20885 Australian Government Bureau of Meteorology Queensland Flood Summary Issued at 9:47 AM on Wednesday the 1st of December 2010 The following Watches/Warnings are current: FLOOD WARNING FOR THE BULLOO RIVER FLOOD WARNING FOR THE WARREGO RIVER FLOOD WARNING FOR THE THOMSON AND BARCOO RIVERS AND COOPER CREEK For more information on flood warnings see: www.bom.gov.au/qld/warnings/ Additional information: Other flooding includes: Diamantina River: Minor flood levels are falling at Diamantina Lakes with minor flooding rising slowly at Monkira. Paroo River: Minor flooding is falling slowly at Hungerford. Moonie River: Minor flooding is rising at Nindigully. Dawson River: Minor flooding is rising at Tarana Crossing. Warnings and River Height Bulletins are available at http://www.bom.gov.au/qld/flood/ . Flood Warnings are also available on telephone 1300 659 219 at a low call cost of 27.5 cents, more from mobile, public and satellite phones. IDQ20885 Australian Government Bureau of Meteorology Queensland Flood Summary Issued at 5:30 PM on Wednesday the 1st of December 2010 The following Watches/Warnings are current: FLOOD WARNING FOR THE BULLOO RIVER FLOOD WARNING FOR THE FITZROY RIVER BASIN FLOOD WARNING FOR THE THOMSON AND BARCOO RIVERS AND COOPER CREEK FLOOD WARNING FOR THE WARREGO RIVER PB2-8(31) 3 Additional information: Other flooding includes: Diamantina River: Minor flood levels are falling at Diamantina Lakes with minor flooding rising at Monkira. -

Permanent Water Trading Interim Report
Permanent Water Trading Interim Report SURFACEWATER, SUPPLEMENTED SUPPLY Period: 1 May 2019 to 31 May 2019 Transfer of Ownership – Water Allocations Water Plan Water Supply Scheme Priority Group Number Of Volume Volume Weighted Transfers Transferred Turnover Average Price (ML) (%) ($/ML) Water Plan (Barron) 2002 MAREEBA DIMBULAH MEDIUM 3 137 <1 3,519 WATER SUPPLY SCHEME Period Total 3 137 <1 3,519 Financial YTD 74 3,618 2 12,437 Water Plan (Border MACINTYRE BROOK MEDIUM 1 5 <1 2,000 Rivers and Moonie) 2019 WATER SUPPLY SCHEME Period Total 1 5 <1 2,000 Financial YTD 12 2,012 2 2,397 Water Plan (Burdekin Financial YTD 16 9,998 <1 624 Basin) 2007 Water Plan (Burnett BARKER BARAMBAH MEDIUM 1 10 <1 1,500 Basin) 2014 WATER SUPPLY SCHEME BUNDABERG WATER MEDIUM 15 1,713 <1 940 SUPPLY SCHEME Period Total 16 1,723 <1 943 Financial YTD 102 8,455 2 3,296 Water Plan (Condamine UPPER CONDAMINE MEDIUM 2 67 <1 2,500 and Balonne) 2019 WATER SUPPLY SCHEME Period Total 2 67 <1 2,500 Financial YTD 25 11,854 10 3,552 Water Plan (Fitzroy NOGOA MACKENZIE MEDIUM 1 800 <1 0 Basin) 2011 WATER SUPPLY SCHEME Period Total 1 800 <1 0 Financial YTD 98 22,035 6 1,617 Water Plan (Logan Basin) LOGAN RIVER MEDIUM 1 5 <1 500 2007 WATER SUPPLY SCHEME Period Total 1 5 <1 500 Financial YTD 6 209 <1 620 Water Plan (Mary Basin) LOWER MARY RIVER MEDIUM 1 30 <1 850 2006 WATER SUPPLY SCHEME Period Total 1 30 <1 850 Financial YTD 35 1,629 1 531 Water Plan (Moreton) CENTRAL BRISBANE MEDIUM 1 80 1 0 2007 RIVER WATER SUPPLY SCHEME WARRILL VALLEY MEDIUM 3 210 <1 0 WATER SUPPLY SCHEME -

Climatically-Controlled River Terraces in Eastern Australia
Article Climatically-Controlled River Terraces in Eastern Australia James S. Daley 1,2,* and Tim J. Cohen 3,4 1 Griffith Centre for Coastal Management, Griffith University, Gold Coast, QLD 4215, Australia 2 School of Earth and Environmental Science, University of Queensland, St. Lucia, QLD 4072, Australia 3 ARC Centre of Excellence for Australian Biodiversity and Heritage–School of Earth and Environmental Sciences, University of Wollongong, Wollongong, NSW 2522, Australia; [email protected] 4 GeoQuEST Research Centre–School of Earth and Environmental Sciences, University of Wollongong, Wollongong, NSW 2522, Australia * Correspondence: j.daley@griffith.edu.au Academic Editors: Jef Vandenberghe, David Bridgland and Xianyan Wang Received: 23 August 2018; Accepted: 13 October 2018; Published: 16 October 2018 Abstract: In the tectonically stable rivers of eastern Australia, changes in response to sediment supply and flow regime are likely driven by both regional climatic (allogenic) factors and intrinsic (autogenic) geomorphic controls. Contentious debate has ensued as to which is the dominant factor in the evolution of valley floors and the formation of late Quaternary terraces preserved along many coastal streams. Preliminary chronostratigraphic data from river terraces along four streams in subtropical Southeast Queensland (SEQ), Australia, indicate regionally synchronous terrace abandonment between 7.5–10.8 ka. All optically stimulated luminescence ages are within 1σ error and yield a mean age of incision at 9.24 ± 0.93 ka. Limited samples of the upper parts of the inset floodplains from three of the four streams yield near-surface ages of 600–500 years. Terrace sediments consist of vertically accreted fine sandy silts to cohesive clays, while top stratum of the floodplains are comprised of clay loams to fine-medium sands.