On the Use of Kriging Model Concept to Detect Groundwater Potential Zones in Hilly Dutsin-Ma, Northwestern Nigeria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
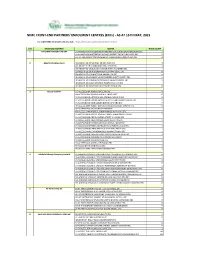
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

Report of the Technical Committee Om
REPORT OF THE TECHNICAL COMMITTEE ON CONSTITUTIONAL PROVISIONS FOR THE APPLICATION OF SHARIA IN KATSINA STATE January 2000 Contents: Volume I: Main Report Chapter One: Preliminary Matters Preamble Terms of Reference Modus Operandi Chapter Two: Consideration of Various Sections of the Constitution in Relation to Application of Sharia A. Section 4(6) B. Section 5(2) C. Section 6(2) D. Section 10 E. Section 38 F. Section 275(1) G. Section 277 Chapter Three: Observations and Recommendations 1. General Observations 2. Specific Recommendations 3. General Recommendations Conclusion Appendix A: List of all the Groups, Associations, Institutions and Individuals Contacted by the Committee Volume II: Verbatim Proceedings Zone 1: Funtua: Funtua, Bakori, Danja, Faskari, Dandume and Sabuwa Zone 2: Malumfashi: Malumfashi, Kafur, Kankara and Musawa Zone 3: Dutsin-Ma: Dutsin-Ma, Danmusa, Batsari, Kurfi and Safana Zone 4: Kankia: Kankia, Ingawa, Kusada and Matazu Zone 5: Daura: Daura, Baure, Zango, Mai’adua and Sandamu Zone 6: Mani: Mani, Mashi, Dutsi and Bindawa Zone 7: Katsina: Katsina, Kaita, Rimi, Jibia, Charanchi and Batagarawa 1 Ostien: Sharia Implementation in Northern Nigeria 1999-2006: A Sourcebook: Supplement to Chapter 2 REPORT OF THE TECHNICAL COMMITTEE ON APPLICATION OF SHARIA IN KATSINA STATE VOLUME I: MAIN REPORT CHAPTER ONE Preamble The Committee was inaugurated on the 20th October, 1999 by His Excellency, the Governor of Katsina State, Alhaji Umaru Musa Yar’adua, at the Council Chambers, Government House. In his inaugural address, the Governor gave four point terms of reference to the Committee. He urged members of the Committee to work towards realising the objectives for which the Committee was set up. -

IOM Nigeria DTM Flash Report NCNW 26 June 2020
FLASH REPORT: POPULATION DISPLACEMENT DTM North West/North Central Nigeria. Nigeria 22 - 26 JUNE 2020 Aected Population: Casualties: Movement Trigger: 2,349 Individuals 3 Individuals Armed attacks OVERVIEW Maikwama 219 The crisis in Nigeria’s North Central and North West zones, which involves long-standing Dandume tensions between ethnic and linguis�c groups; a�acks by criminal groups; and banditry/hirabah (such as kidnapping and grand larceny along major highways) led to fresh wave of popula�on displacement. Kaita Mashi Mai'adua Jibia Shinkafi Katsina Daura Zango Dutsi Batagarawa Mani Safana Latest a�acks affected 2,349 individuals, includ- Zurmi Rimi Batsari Baure Maradun Bindawa Kurfi ing 18 injuries and 3 fatali�es, in Dandume LGA Bakura Charanchi Ingawa Jigawa Kaura Namoda Sandamu Katsina Birnin Magaji Kusada Dutsin-Ma Kankia (Katsina) and Bukkuyum LGA (Zamfara) between Talata Mafara Bungudu Matazu Dan Musa 22 - 26 June, 2020. The a�acks caused people to Gusau Zamfara Musawa Gummi Kankara flee to neighboring locali�es. Bukkuyum Anka Tsafe Malumfashi Kano Faskari Kafur Gusau Bakori A rapid assessment was conducted by field staff Maru Funtua Dandume Danja to assess the impact on people and immediate Sabuwa needs. ± GENDER (FIG. 1) Kaduna X Affected PopulationPlateau 42% Kyaram 58% Male State Bukkuyum 2,130 Female Federal Capital Territory LGA Nasarawa Affected LGAs The map is for illustration purposes only. The depiction and use of boundaries, geographic names and related data shown are not warranted to be error free nor do they imply judgment on the legal status of any territory, or any endorsement or accpetance of such boundaries by MOST NEEDED ASSISTANCE (FIG. -

2018/2019 Annual School Census Report
Foreword Successful education policies are formed and supported by accurate, timely and reliable data, to improve governance practices, enhance accountability and ultimately improve the teaching and learning process in schools. Considering the importance of robust data collection, the Planning, Research and Statistics (PRS) Department, Katsina State Ministry of Education prepares and publishes the Annual Schools Census Statistical Report of both Public and Private Schools on an annual basis. This is in compliance with the National EMIS Policy and its implementation. The Annual Schools Census Statistical Report of 2018-2019 is the outcome of the exercise conducted between May and June 2019, through a rigorous activities that include training Head Teachers and Teachers on School Records Keeping; how to fill ASC questionnaire using school records; data collection, validation, entry, consistency checks and analysis. This publication is the 13th Annual Schools Census Statistical Report of all Schools in the State. In line with specific objectives of National Education Management Information System (NEMIS), this year’s ASC has obtained comprehensive and reliable data where by all data obtained were from the primary source (the school’s head provide all data required from schools records). Data on Key Performance Indicators (KPIs) of basic education and post basic to track the achievement of the State Education Sector Operational Plan (SESOP) as well as Sustainable Development Goals (SDGs); feed data into the National databank to strengthen NEMIS for national and global reporting. The report comprises of educational data pertaining to all level both public and private schools ranging from pre-primary, primary, junior secondary and senior secondary level. -

Biodiversity: Management and Control As a Catalyst for Environmental Sustainability in Katsina State, Nigeria
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 19, Issue 8, Ver. IV (Aug. 2014), PP 31-35 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org Biodiversity: Management and Control as a Catalyst for Environmental Sustainability in Katsina State, Nigeria AminuRilwanu Kankara Department of Geography, Isa Kaita College of Education, PMB. 5007, Dutsinma, Katsina State, Nigeria Abstract: The variety of ecosystems, species and genes that surround us, is our life insurance, giving us food, fresh water and clean air, shelter and medicine, mitigating natural disasters, pests and diseases and contributes in mitigating effects of climate change. Biodiversity is also our natural capital, delivering ecosystem services that underpin our economy. In this research work the researcher was able to identify some devastating effects due to loss of the natural biodiversity. Research also identifies some measures or strategies used in combating such menace of biodiversity loss. The research work was able to identify some of the significant issues of maintaining the natural biodiversity. Moreover, findings show that; loss of biodiversity may leads to rainfalls shortageand lower the volume of water table, general extinction of forest and animals. It may also cause soil degradation, loss of soil fertility, soil erosion, and desertification as well as food shortage. Finally, the researcher suggested some measures for minimizing the negative impact of biodiversity loss. Such measures can be done through controlling grazing, afforestation program and through use of alternative source of fuel. Keywords: Biodiversity, Management and Environmental sustainability I. Introduction Biodiversity: the term used to indicate the distribution of the variety of ecosystems, species and genes that surround our natural environment. -

Curriculum Vitae
CURRICULUM VITAE CONTACT ADDRESS: C/O Dept. of Geography and Regional Planning, Federal University Dutsin-Ma, PMB 5001, Katsina State, Nigeria HOME ADDRESS: No. 5, Tudun-Matawalle Quarters, Katsina City, Nigeria GSM: 07030797630 Email: [email protected] PERSONAL DATA: ~Name IBRAHIM, Aliyu ~Place of Birth Kankara ~State of Birth Katsina ~Date of Birth 20th Dec, 1968 ~Nationality Nigerian ~Local Government Kankara ~Marital Status Married ~Number and ages of children 4/ 9, 7, 3, 1 ~Rank Senior Lecturer ~Grade Level CONUASS 5/2 ~Religion Islam Occupation: Lecturing Date of first appointment: 26/12/2006 Date of present appointment 16/12/2014 Extra Curricular Activities: Writing, Travelling and Interacting with People EDUCATIONAL INSTITUTIONS ATTENDED WITH DATES: ~ Federal University of Technology, Minna 2007 --- 2014 ~ Bayero University Kano 1999 --- 2003 ~ Ahmadu Bello University Zaria 1990 --- 1994 ~ College of Advanced Studies Zaria 1986 --- 1990 ~ Government College Katsina 1981 --- 1986 ~ Nuhu Primary School Kankara 1975 --- 1981 ACADEMIC QUALIFICATIONS OBTAINED WITH DATES: ~ Doctorate Degree in Geology (Economic Geology) 2014 ~ Msc. Land Resources Development) 2003 ~ Bsc. (Hons.) Geology 1994 ~ IJMB (Result) 1990 ~ West African School Certificate 1986 ~ First School Leaving Certificate 1981 ADDITIONAL EDUCATION/QUALIFICATIONS WITH ADDRESSES AND DATE Certificate course in Computer Appreciation at BH Computers Katsina, 2009 1 WORKING EXPERIENCE S/N Position Employer Duration/Year 1 Dept. of Geography and Regional Senior Planning, -

Appropriation to Katsina State in the 2012 National Budget
APPROPRIATION TO KATSINA STATE IN THE 2012 NATIONAL BUDGET MINISTRY/DEPARTMENT/ PROJECT LOCATION AMOUNT AGENCY NATIONAL POVERTY ERADICATION POVERTY REDUCTION GRANTS SCHEME IN MALUMFASHI/KAFUR FEDERAL MALUMFASHI/ KAFUR, KATSINA 50,000,000 CONSTITUENCY, KATSINA STATE. PROGRAMME, NAPEP (OFFICE OF THE POVERTY ALLEVIATION/YOUTH EMPOWERMENT IN 11 LGA, KATSINA SOUTH 200,000,000 SECRETARY TO THE GOVERNMENT OF SENATORIAL DISTRICT, KATSINA STATE THE FEDERATION) FEDERAL MINISTRY OF YOUTH & YOUTH DEVELOPMENT CENTRE-KATSINA KATSINA 49,000,000 SOCIAL DEVELOPMENT FEDERAL MINISTRY OF WATER CONSTRUCTION / PROVISION OF WATER FACILITIES ZOBE KATSINA 61,364,400 IRRIGATION PROJECT RESOURCES CONSTRUCTION / PROVISION OF SABKE IRRIGATION PROJECT (PTF TRANSFERRED PROJECT). 1,435,367 WATER FACILITIES REHABILITATION AND EXPANSION OF JIBIA IRRIGATION SCHEME BY 1000 HA 100,000,000 ZOBE WATER SUPPLY PROJECT - PHASE II 20,000,000 CONSTRUCTION OF SULMA DAM 4,559,317 CONSTRUCTION OF MASHI DAM 5,942,942 CONSTRUCTION OF JARE EARTH DAM 10,040,000 SOKOTO RIMA RIVER BASIN CONSTRUCTION OF MINOR IRRIGATION SCHEME AT 11 LGA, KATSINA SOUTH KATSINA 300,000,000 SENATORIAL DISTRICT, KATSINA STATE. DEVELOPMENT AUTHORITY CONSTRUCTION OF DAM AT MATAZU LGA, PANDABUWA/MATAZU DAM 150,000,000 KATSINA STATE. CONSTRUCTION OF DAM AT KAFUR LGA, SULMA DAM KATSINA STATE. 100,000,000 REHABILITATION AND PROCUREMENT OF EQUIPMENT FOR BAKOLORI 100,000,000 IRRIGATION PROJECT (SRRBDA) MINOR IRRIGATION SCHEME AT KOKO BESSE, DUTSHINMARI, ZAR AKALA-KALA, 25,000,000 JADADI, LANI CONSTRUCTION OF BOREHOLES -

The State and Ecological Problems in Katsina, Nigeria
• A rican Arid Lands Working Paper Series ISSN 1102-4488 NORDISKA AfR\KAINSTITUiEi 1B 2 -02- , , UPPSA\.A Nordiska Afrikainstitutet (The Scandinavian Institute of African Studies) p O Box 1703, S-751 47 UPPSALA, Sweden Telex 8195077, Telefax 018-69 56 29 African Arid Lands Working Paper Series is published within the Nordiska Mrikainstitutet research prograrnrne Human Life in African Arid Lands, the main objectives of which are as follows: to encourage research in the drylands and the exchange of country and regional experiences, and to link up with the ongoing activities in Mrica and the Nordic countries to enhance the cooperation between social and natural science disciplines and indigenous knowledge to explore the hearing of developmental policies on the environment and the possibility of devising long-term strategies for the redemption of the fragile drylands environment Editorial staff: Anders Hjort af Ornäs, Director of Nordiska Afrikainstitutet M.A. Mohamed Salih, Prograrnrne Leader of Human Life in African Arid Lands Eva Lena Volk, Prograrnrne Assistant, Human Life in African Arid Lands illustration on front: Details from a decorated gourd (in Nigeria 's Traditional Crafts by Alison Hodge) A/riean Arid Lands Working Paper Series No. 1/92 THE STATE AND ECOLOGICAL PROBLEMS IN KATSINA, NIGERIA by A. M. SAULAWA Department of History, Usmanu Danfodiyo University, Sokoto, Nigeria. ABSTRACT An important feature in the Nigerian and African past and recent history has been the environment and its continuous inf1uence on the population. This issue is one that is much tied up with the ecological problems and the challenges they pose to the population. -

Katsina State of Nigeria State Education Sector Strategic Plan (Sesp)
KATSINA STATE OF NIGERIA STATE EDUCATION SECTOR STRATEGIC PLAN (SESP): 2011 – 2020 SEPTEMBER, 2010. 1 | Page Table of Contents INTRODUCTION: Preamble 4 Section A: 5 a. Katsina State Socio-economic, geographical and political highlights 5 b. Major Highlights of Education in Katsina State 6 c. Persistent Challenges of the Education Sector 7 d. Need for Sector Plan. e. Process for Developing the Sector Plan Section B: (Major Challenge Areas) 10 a. Inadequate coverage and unsatisfactory level of meaningful access 13 b. Poor quality and relevance 20 c. Infrastructural insufficiency and infrastructural decay 24 d. Inefficient management and system inefficiency 30 e. Non-sustainable funding and inadequate resourcing 31 Section C: (Management of the Process of Plan Execution) 31 . List of Acronyms 32 ANNEX (Vital Education Statistics) 33 2 | Page 3 | Page Preamble The Katsina State Education Sector Strategic Plan (SESP) is aimed at articulating the education vision of the State Government. It is hoped that the plan will give some concrete directions to the government’s determination by addressing the many challenges of the Education Sector. The process of the 10-year Strategic plan and its 3-year operational plan was achieved through a participatory process which was effectively led by the government and people of the state. The process involved the active participation of stakeholders who contributed rigorously in the analysis of the Education Sector and in charting the course of the future development. These future developments emphasize strategic targets to be met in a 10-year perspective (2010- 2020) which addressed five major challenge areas, namely; 1. Inadequate coverage and unsatisfactory level of meaningful access, 2. -

Prevalence of Trachoma in Katsina State, Nigeria: Results of 34 District-Level Surveys
Ophthalmic Epidemiology ISSN: 0928-6586 (Print) 1744-5086 (Online) Journal homepage: http://www.tandfonline.com/loi/iope20 Prevalence of Trachoma in Katsina State, Nigeria: Results of 34 District-Level Surveys Caleb Mpyet, Nasiru Muhammad, Mohammed Dantani Adamu, Habila Muazu, Murtala Mohammad Umar, Musa Goyol, Uwazoeke Onyebuchi, Ima Chima, Haliru Idris, Adamani William, Sunday Isiyaku, Benjamin Nwobi, Rebecca Mann Flueckiger, Rebecca Willis, Alexandre Pavluck, Brian K. Chu, Nicholas Olobio & Anthony W. Solomon To cite this article: Caleb Mpyet, Nasiru Muhammad, Mohammed Dantani Adamu, Habila Muazu, Murtala Mohammad Umar, Musa Goyol, Uwazoeke Onyebuchi, Ima Chima, Haliru Idris, Adamani William, Sunday Isiyaku, Benjamin Nwobi, Rebecca Mann Flueckiger, Rebecca Willis, Alexandre Pavluck, Brian K. Chu, Nicholas Olobio & Anthony W. Solomon (2016) Prevalence of Trachoma in Katsina State, Nigeria: Results of 34 District-Level Surveys, Ophthalmic Epidemiology, 23:sup1, 55-62, DOI: 10.1080/09286586.2016.1236975 To link to this article: https://doi.org/10.1080/09286586.2016.1236975 Published with license by Taylor & Francis© Published online: 24 Oct 2016. 2016 Caleb Mpyet, Nasiru Muhammad, Mohammed Dantani Adamu, Habila Muazu, Murtala Mohammad Umar, Musa Goyol, Uwazoeke Onyebuchi, Ima Chima, Haliru Idris, Adamani William, Sunday Isiyaku, Benjamin Nwobi, Rebecca Mann Flueckiger, Rebecca Willis, Alexandre Pavluck, Brian K. Chu, Nicholas Olobio, and Anthony W. Solomon, for the Global Trachoma Mapping Project. Submit your article to this journal Article views: 303 View related articles View Crossmark data Citing articles: 3 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=iope20 OPHTHALMIC EPIDEMIOLOGY 2016, VOL. 23, NO. -

1St Admission List 2021
DEPARTMENT OF COMPUTER SOFTWARE ENGINEERING FIRST ADMISSION LIST 2020/2021 ACADEMIC SESSION S/N NAME JAMB NO LG STATE REMARK 1 DALHATU IBRAHIM 21020422JF KATSINA KATSINA 2 AUWAL YUSUF 21136838IA KATSINA KATSINA 3 IBRAHIM AMINU BINDAWA 22199618AF BINDAWA KATSINA 4 ABDULHAKIM RABE KAITA 21106916GF KAITA KATSINA 5 ZAINAB SHITU 21107622FA KATSINA KATSINA 6 ISMAIL ABUBAKAR 22199618FA KAFUR KATSINA 7 ISMAIL YUSUF YUSUF 20990054DA GUMEL JIGAWA 8 ISA SADA BOKO 22315843JA KUSADA KATSINA 9 MUHAMMAD BELLO ALIYU 21106499GA MALUMFASHI KATSINA 10 MAIMUNA RABIU LAWAL 21127855GA KATSINA KATSINA 11 MUSA ABDULLAHI 21125117HA BINDAWA KATSINA 12 ABDURAZAK KABIR 21116449JA INGAWA KATSINA 13 MUHAMMAD AUWAL IBRAHIM 21110744CA CHARANCHI KATSINA 14 MUSTAPHA SURAJO 21110284EA DANMUSA KATSINA 15 MUSTAPHA UMAR 21134230BA KATSINA KATSINA 16 FATIMA KABIR HASHIM 21121067IF KATSINA KATSINA 17 MUHAMMAD S. YAKUBU 21110991CA KATSINA KATSINA 18 IBRAHIM ABUBAKAR 21127872HA BATAGARAWA KATSINA 19 JIBRIL ABUBAKAR BINDAWA 21110188EE BINDAWA KATSINA 20 JUWAIRIYYA IBRAHIM SAULAWA 21135412IF KATSINA KATSINA 21 MUSA RABI'U 21126673IA KATSINA KATSINA 22 ABDULAZIZ ABUBAKAR 2111000IDA KATSINA KATSINA 23 IBRAHIM KABIR NAKOWA 21135243HF BINDAWA KATSINA 24 AHMAD S. ADAMU 21117503EF KATSINA KATSINA 25 AHMAD ZAILANI 21111363GA DANJA KATSINA 26 UMAR MUHAMMAD 22141172BF BATAGARAWA KATSINA 27 LAWAL ABUBAKAR HALILU 21107372GF KATSINA KATSINA 28 ABUBAKAR ABDULKADIR 22200469CF KURFI KATSINA 29 SALISU ALIYU 21134584EF BATAGARAWA KATSINA 30 YUSUF MANSUR 21133259CA KATSINA KATSINA 31 MUSA MUSA 21127197CF -
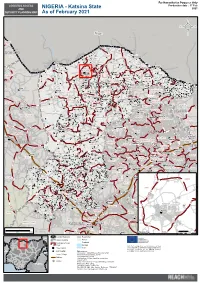
Katsina State 2021 SECURITY PLANNING MAP As of February 2021
For Humanitarian Purposes Only LOGISTICS ACCESS Production date : 17 Feb AND NIGERIA - Katsina State 2021 SECURITY PLANNING MAP As of February 2021 Niger SABON BIRNI Dutsin Bamli Forest* Dan Kunama Dan Kabba SOKOTO Forest Re Dagura Dadin Sarki ISA Matsai Maiaduwa KAITA Washemi Jibiya MAI'ADUA Jasai Kaita MASHI Mazanya Jerdedi Forest Zango Rese* Daura JIBIA Karawa Gulbin Baure KATSINA Forest* Zandam, Gulbi ZANGO Katsina Mashi Sandamu DAURA Forest Dan Tambara Fiwuni Kututure SHINKAFI Katsina Damakola Rese* Gurjiya Kigo, River Sandamu DUTSI Sukanawa MANI Unguwar GajeUnguwar Gaje Batagarawa Kaurin Saidu Pakarawa Forest* Maikiliya Dan Aunai SANDAMU Yantuba Dutsawa Lemo Tamdamwa Dutsawa Sabon Gari Fulani Giginya Nat* Forest Gidan Adamu Burudu Gargarawa Rese* Saboa Dandogo Rimi Mani Forest* Maibara Rimi Baure DancaleDancale Dutsi Kufani Adalawa Salei BATSARI Kawari Bardaya Zakata Dutsi BAURE Zurmi Gwiwa Kyaras ZURMI Yan Molo Batsari Alumu RIMI BINDAWA YANKWASHI Inga Dole GWIWA Musari Ungua Tsamia Tuwaru Sabuwa Dan KuliyaDan Gagi Daura Kuraye Yankwashi Kwahi Bagadi Safana Kabura Gawaji Kuma Faru Tachiawa Kurfi Yanuku Charanchi Roni Kazaure Ajiwa Bindawa Ingawa KURFI CHARANCHI Dandi Lake Gaiwa KAURA Dan Jakko Dambuna KAZAURE INGAWA Ruruma Birci NAMODA Kirkini Yakassa Jobe BABURA Gamda RONI Birni Mogaji Kankiya Gaci Kunchi MAKODA SAFANA Daddare Eaguwa Forest Rese JIGAWA Kusada BIRNIN MAGAJI Dutsin Ma KUSADA Dambatta Kafarda KUNCHI Safana KANKIA Makoda DAMBATTA GARKI Magwai, River Ungwan Adar Duru Ganuwa Dan-Tudu Makanwashi Karaduwa Jigawar Sawai