Duplex Sonography of the Temporal and Occipital Artery in the Diagnosis of Temporal Arteritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurovascular Anatomy (1): Anterior Circulation Anatomy
Neurovascular Anatomy (1): Anterior Circulation Anatomy Natthapon Rattanathamsakul, MD. December 14th, 2017 Contents: Neurovascular Anatomy Arterial supply of the brain . Anterior circulation . Posterior circulation Arterial supply of the spinal cord Venous system of the brain Neurovascular Anatomy (1): Anatomy of the Anterior Circulation Carotid artery system Ophthalmic artery Arterial circle of Willis Arterial territories of the cerebrum Cerebral Vasculature • Anterior circulation: Internal carotid artery • Posterior circulation: Vertebrobasilar system • All originates at the arch of aorta Flemming KD, Jones LK. Mayo Clinic neurology board review: Basic science and psychiatry for initial certification. 2015 Common Carotid Artery • Carotid bifurcation at the level of C3-4 vertebra or superior border of thyroid cartilage External carotid artery Supply the head & neck, except for the brain the eyes Internal carotid artery • Supply the brain the eyes • Enter the skull via the carotid canal Netter FH. Atlas of human anatomy, 6th ed. 2014 Angiographic Correlation Uflacker R. Atlas of vascular anatomy: an angiographic approach, 2007 External Carotid Artery External carotid artery • Superior thyroid artery • Lingual artery • Facial artery • Ascending pharyngeal artery • Posterior auricular artery • Occipital artery • Maxillary artery • Superficial temporal artery • Middle meningeal artery – epidural hemorrhage Netter FH. Atlas of human anatomy, 6th ed. 2014 Middle meningeal artery Epidural hematoma http://www.jrlawfirm.com/library/subdural-epidural-hematoma -

The Ascending Pharyngeal Artery: a Collateral Pathway in Complete
AJNR :8, January/February 1987 CORRESPONDENCE 177 cavernous sinuses acute inflammation, granulation tissue, and throm which it partiCipates. This report describes two cases in which bus surrounded the nerves and internal carotid arteries. The left common carotid angiography showed complete occlu sion of the carotid artery was intact, but focally inflammed. The right internal internal carotid artery at its origin. Subsequent vertebral angiography carotid artery was focally necrotic, acutely inflammed and ruptured, in both cases showed reconstitution of thi s vessel several millimeters with hemorrhage emanating from the defect. above the origin by the ascending ph aryngeal artery , which had an unusual origin from the internal carotid artery [2]. Endarterectomy as a technical option was feasible in both cases becau se the occluded Discussion segments were only millimeters in length . The first patient, a 59-year-old man , presented 5 days before We are not aware of any instances of air within the cavernous admission with a sudden pareS is of the right arm and leg. Angiograph y sinus in a normal patient or after trauma. Our case demonstrates revealed complete occlusion of the left internal carotid artery with a several of the reported findings in cavernous sinus thrombosis includ small , smooth stump (Fig. 1 A) . A left vertebral arteriogram demon ing bulging of the lateral walls , irregular low-attenuation filling defects strated reconstitution of the left internal carotid artery just above the within the cavernous sinus, and proptosis (Fig . 1). occlusion (Fig . 1 B). Collateral supply was from mu scular branches of It is unclear whether the air within the sinus originated from a gas the vertebral artery, which anastomosed with muscular branches of forming organism or via direct extension from one of the sinuses via the ascending pharyngeal artery. -

Penetrating Vascular Injuries of the Face and Neck: Clinical and Angiographic Correlation
855 Penetrating Vascular Injuries of the Face and Neck: Clinical and Angiographic Correlation Charles M. North 1. 2 A retrospective review was made of 139 clinically stable patients who had sustained Jamshid Ahmadi penetrating trauma to the face and neck. The study was done to learn more about the Hervey D. Segall indications for angiography and the impact of angiography upon patient management. Chi-Shing Zee Some relationship between the physical examination and the angiographic findings was found. In the presence of anyone of four physical signs or symptoms (absent pulse, bruit, hematoma, or alteration of neurologic status) there was a 30% incidence of vascular injury. However, it is unlikely that a clinically significant traumatic vascular lesion will be missed if angiography is not obtained when these clinical signs and symptoms are not present. In the group of 78 patients who presented with only a wound penetrating the ' platysma and no other findings or symptoms, just two had vascular injuries on angiograms; one of these lesions was minor and the other did not affect the patient's management. There was a substantially higher rate (50%) of vascular injury in patients with trauma cephalad to the angle of the mandible compared with 11 % of patients who had neck trauma. Gunshot wounds were associated with vascular damage more frequently than were stab wounds. Angiography is often performed in penetrating trauma to the head and neck to evaluate the possibility of vascular injury and to aid in planning appropriate management [1]. Nonetheless, the role of angiography in penetrating head and neck trauma has remained controversial. -

Dural Arteriovenous Malformation of the Major Venous Sinuses: an Acquired Lesion
13 Dural Arteriovenous Malformation of the Major Venous Sinuses: An Acquired Lesion Mohammad Y. Chaudhary,1.2 Arteriovenous malformations of the dura are thought to be congenital. However, Ved P. Sachdev3 arteriographic investigations of four patients who, after a head injury, developed dural Soo H. Ch01 arteriovenous fistulae with features of congenital malformations suggest that these Imre Weitzner, Jr.1 abnormal communications may also be acquired. Thrombosis or thrombophlebitis in Smiljan Puljic2 the dural sinus or vein may be the primary event in their formation. The pathogenesis Yun Peng Huang 1 is probably " growth" of the dural arteries normally present in the walls of the sinuses during the organization of an intraluminal thrombus. This may result in a direct communication between artery and vein or sinus, establishing an abnormal shunt. Ultimate fibrosis of the sinus wall and intraluminal thrombus may be the factors responsible for the spontaneous disappearance of such malformations. Most dural arteriovenous malformations (AVMs) that involve th e major venous sinuses present either spontaneously or as incidental findings during arteriog raphy performed for other reasons. They occur predominantly in women over age 40 years [1]. The angiomatous network, multiple feeding arteries, numerous arteriovenous (A V) shunts, and occasional association with cerebral angiomas [2], as well as a few cases reported in children [3], suggest that these AVM s are congenital. Thrombosis of the draining sinus or vein is thought to be responsible for the occasional spontaneous disappearance of these lesions [4, 5]. Our experience with four patients who, after a head injury, developed dural AV fistulae with features of congenital malformations prompted a review of th e literature and this report. -
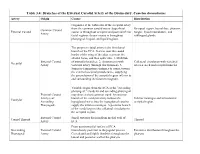
Branches of the External Carotid Artery of the Dromedary, Camelus Dromedarius Artery Origin Course Distribution
Table 3.4: Branches of the External Carotid Artery of the Dromedary, Camelus dromedarius Artery Origin Course Distribution Originates at the bifurcatio of the occipital artery from the common carotid artery. Superficial Occipital region, lateral face, pharynx, Common Carotid External Carotid course is throughout occipital and posteroinferior tongue, hyoid musculature, and Artery facial regions; deeper course is throughout sublingual glands. pharyngeal, lingual, and hyoid regions. The proper occipital artery is the first dorsal branch of the ECA. It arises near the caudal border of the wing of the atlas, traverses the atlantal fossa, and then splits into: 1. Multitude External Carotid of muscular branches; 2. Anastomosis with Collateral circulation with vertebral Occipital Artery vertebral artery (through alar foramen); 3. arteries; neck and occipital muscles Superior termination continues to course toward the external occipital protuberance, supplying the parenchyma of the occipital region inferior to and surrounding the foramen magnum. Variable origin: from the ECA or the "ascending pharyngeal." Condylar and ascending pharyngeal External Carotid may share a short common trunk. An anterior Artery (var: branch of the condylar artery follows the Inferior meninges and inferolateral Condylar Ascending hypoglossal nerve into the hypoglossal canal to occipital region. Pharyngeal) supply the inferior meninges. A posterior branch of the condylar provides collateral circulation to the occipital region. External Carotid Small, tortuous division from medial wall of Cranial Thyroid Thyroid Artery ECA From posteromedial surface of ECA Descending External Carotid immediately posterior to the jugular process. Extensive distribution throughout the Pharyngeal Artery Convoluted and highly dendritic throughout the pharynx lateral and posterior wall of the pharynx. -

The Human Central Nervous System
The Human Central Nervous System A Synopsis and Atlas Bearbeitet von Rudolf Nieuwenhuys, Jan Voogd, Christiaan van Huijzen 4th ed. 2007. Buch. xiv, 967 S. Hardcover ISBN 978 3 540 34684 5 Format (B x L): 20,3 x 27,6 cm Weitere Fachgebiete > Psychologie > Allgemeine Psychologie / Grundlagenfächer > Biologische Psychologie, Neuropsychologie, Psychophysiologie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 4 Blood Supply, Meninges and Cerebrospinal Fluid Circulation Introduction......................... 95 through the arachnoid villi to the venous sys- ArteriesoftheBrain................... 95 tem. The nervous tissue of the central nervous Meninges, Cisterns system and the CSF spaces remain segregated and Cerebrospinal Fluid Circulation ........110 from the rest of the body by barrier layers in Circumventricular Organs ................126 the meninges (the barrier layer of the arach- Veins of the Brain .....................126 noid), the choroid plexus (the blood-CSF bar- Vessels and Meninges of the Spinal Cord .....128 rier) and the capillaries (the blood-brain bar- rier). The circulation of the CSF plays an impor- tant role in maintaining the environment of the nervous tissue; moreover, the subarachnoidal space forms a bed that absorbs external shocks. Introduction The vascularization and the circulation of the Arteries of the Brain cerebrospinal fluid (liquor cerebrospinalis, CSF) of the brain and the spinal cord are of great clinical importance. -

The 0Ccipital-Vertebral Anastomosis
The 0ccipital-Vertebral Anastomosis MANNIE M. SCHECIITER,M.D. Section of Neuroradiology, Department of Radiology, Albert Einstein College of Medicine, New York, New York HE presence and significance of collat- artery. In the past this was, in fact, the basis eral circulation between the various for techniques of indirect vertebral angiog- T branches of the intracranial circulation raphy in which the right carotid artery was and branches of the intracranial and extra- compressed distal to the site of the puncture cranial circulation have been described in the during angiography.4,5 Similarly retrograde literature. With the current interest and em- carotid catheterization may also be used to phasis in the medical and surgical treatment demonstrate the vertebral artery and its of cerebrovascular disease and with improve- branches).1~ ments in diagnostic procedures, a clearer When filling of the vertebral artery occurs demonstration of these collateral channels is during the injection of contrast medium into now more frequently sought and recognized. the carotid artery or vice versa, the occipital- Most of these potential collateral channels vertebral anastomosis may be demonstrated become obvious only when occlusive vascular by including the cervical course of the verte- disease interrupts the normal pathways, and bral artery in the film. Absence of contrast the channels dilate to form alternate routes medium in the proximal portion of the com- for the passage of blood to vital areas. A mon carotid artery and vertebral artery will temporary differential in the hydrodynamics be recognized readily, excluding this as the of two opposing systems may also reverse the possible course of flow (Figs. -

Anatomy of the Middle Meningeal Artery
Published online: 2021-08-03 THIEME Review Article | Artigo de Revisão Anatomy of the Middle Meningeal Artery Anatomia da artéria meníngea média Marco Aurélio Ferrari Sant’Anna1 Leonardo Luca Luciano2 Pedro Henrique Silveira Chaves3 Leticia Adrielle dos Santos4 Rafaela Gonçalves Moreira5 Rian Peixoto6 Ronald Barcellos7,8 Geraldo Avila Reis7,8 Carlos Umberto Pereira8 Nícollas Nunes Rabelo9 1 Hospital Celso Pierro, Pontifícia Universidade Católica de Address for correspondence Nicollas Nunes Rabelo, MD, Department Campinas, Campinas, SP, Brazil of Neurosurgery, Faculdade Atenas, Passos, Minas Gerais, Rua Oscar 2 School of Medicine, Universidade Federal de Alfenas, Alfenas, MG, Cândido Monteiro, 1000, jardim Colégio de Passos, Passos, MG, Brazil 37900, Brazil (e-mail: [email protected]). 3 Centro Universitário Atenas, Paracatu, MG, Brazil 4 Universidade Federal do Sergipe, Aracaju, SE, Brazil 8 Neurosurgery Department of the Fundação de Beneficência 5 Faculdade Atenas, Passos, MG, Brazil Hospital de Cirurgia Aracaju, SE, Brazil 6 School of Medicine, Faculdade Santa Marcelina, São Paulo, SP, Brazil 9 Neurosurgery Department, Neurosurgery Service of HGUSE and 7 Neurosurgery Department of the Hospital de Urgência de Sergipe the Benefit Foundation Hospital of Surgery, Aracaju, SE, Brazil Governador João Alves Filho, Aracaju, SE, Brazil 10Department of Neurosurgery, Faculdade Atenas, Passos, MG, Brazil Arq Bras Neurocir Abstract Introduction The middle meningeal artery (MMA) is an important artery in neuro- surgery. As the largest branch of the maxillary artery, it provides nutrition to the meninges and to the frontal and parietal regions. Diseases, including dural arteriove- nous fistula (DAVF), pseudoaneurysm, true aneurysm, traumatic arteriovenous fistula (TAVF), Moya-Moya disease (MMD), recurrent chronic subdural hematoma (CSDH), migraine, and meningioma, may be related to the MMA. -

Microsurgical Anatomy of the Dural Arteries
ANATOMIC REPORT MICROSURGICAL ANATOMY OF THE DURAL ARTERIES Carolina Martins, M.D. OBJECTIVE: The objective was to examine the microsurgical anatomy basic to the Department of Neurological microsurgical and endovascular management of lesions involving the dural arteries. Surgery, University of Florida, Gainesville, Florida METHODS: Adult cadaveric heads and skulls were examined using the magnification provided by the surgical microscope to define the origin, course, and distribution of Alexandre Yasuda, M.D. the individual dural arteries. Department of Neurological RESULTS: The pattern of arterial supply of the dura covering the cranial base is more Surgery, University of Florida, complex than over the cerebral convexity. The internal carotid system supplies the Gainesville, Florida midline dura of the anterior and middle fossae and the anterior limit of the posterior Alvaro Campero, M.D. fossa; the external carotid system supplies the lateral segment of the three cranial Department of Neurological fossae; and the vertebrobasilar system supplies the midline structures of the posterior Surgery, University of Florida, fossa and the area of the foramen magnum. Dural territories often have overlapping Gainesville, Florida supply from several sources. Areas supplied from several overlapping sources are the parasellar dura, tentorium, and falx. The tentorium and falx also receive a contribution Arthur J. Ulm, M.D. from the cerebral arteries, making these structures an anastomotic pathway between Department of Neurological Surgery, University of Florida, the dural and parenchymal arteries. A reciprocal relationship, in which the territories Gainesville, Florida of one artery expand if the adjacent arteries are small, is common. CONCLUSION: The carotid and vertebrobasilar arterial systems give rise to multiple Necmettin Tanriover, M.D. -

A Cadaver Study
ORIGINAL ARTICLE The Vascular Anatomy and Angiosome of the Posterior Auricular Artery A Cadaver Study LCDR Brian J. McKinnon, MC, USN; CDR Maryann P. Wall, MC, USN; CDR Daniel W. Karakla, MC, USN Background: Pedicled flaps based on the posterior au- able course in the posterior auricular sulcus. The branch- ricular artery have been used for small auricular and mas- ing pattern over the auricle and temporal bone and the toid cavity defects. artery’s relationship to bony and soft tissue landmarks were consistent. The angiosome includes the anterior and Objective: To precisely define the vascular anatomy and posterior surfaces of the auricle and the periauricular skin angiosome (cutaneous distribution) of the posterior au- superiorly, posteriorly, and inferiorly. ricular artery. Conclusions: The investigation documented the consis- Methods: A fresh cadaver model was used for 3 separate tent vascular anatomy and angiosome of the posterior au- investigations, studying the posterior auricular artery. Intra- ricular artery. The cutaneous distribution suggests that a arterial ink injections defined the angiosome, and subtrac- large pedicled or island flap based on the posterior auricu- tion angiography and latex injection defined the vascular lar artery may be raised safely as a myocutaneous or myo- anatomy in relation to bony and soft tissue landmarks. fasciocutaneous flap with temporalis fascia and/or perios- teum, extending previously published dimensions. Further Subjects: Eight fresh cadavers, 6 men and 2 women, were studies may extend the clinical application to include free used, varying in age from 58 to 85 years. flaps based on the posterior auricular artery. Results: The posterior auricular artery has a predict- Arch Facial Plast Surg. -

A Phylogenetic and Ontogenetic Perspective of the Unique Accumulation of Arterial Variations in One Human Anatomic Specimen
medicina Article A Phylogenetic and Ontogenetic Perspective of the Unique Accumulation of Arterial Variations in One Human Anatomic Specimen Bettina Pretterklieber * and Michael L. Pretterklieber Division of Anatomy, Center for Anatomy and Cell Biology, Medical University of Vienna, A-1090 Vienna, Austria; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 1 September 2020; Published: 4 September 2020 Abstract: Background and objectives: Anatomical dissection is an indispensable means of acquiring knowledge about the variability of the human body. We detected the co-existence of several arterial variations within one female anatomic specimen during routine anatomical dissection. The aim of this study was to evaluate if this status is a regular pattern in any of other vertebrates. Materials and Methods: Besides of a meticulous anatomic dissection, we performed a literature review concerning the frequency, the phylogenesis, and ontogenesis of all of these variations. Results: Exceptionally, the middle colic artery arose from an extraordinarily divided celiac trunk. The kidneys received three polar arteries. On the left side, a corona mortis replaced the obturator artery. The aortic arch gave rise to a bicarotid trunk, and the right subclavian artery originated and coursed as a typical lusorial artery leading to a non-recurrent laryngeal nerve on the right side. Furthermore, variations of the branches of the thyrocervical trunk were found to be present. Extraordinarily, in their cervical portion both internal carotid arteries gave rise to two arteries each. All of these variations developed within two to three weeks, around the sixth week of gestation. It was not possible to ascribe all or even one of the variations to a singular species of vertebrates. -

Anatomy of the Feeding Arteries of the Cerebral Arteriovenous Malformations B
Folia Morphol. Vol. 77, No. 4, pp. 656–669 DOI: 10.5603/FM.a2018.0016 O R I G I N A L A R T I C L E Copyright © 2018 Via Medica ISSN 0015–5659 www.fm.viamedica.pl Anatomy of the feeding arteries of the cerebral arteriovenous malformations B. Milatović1, J. Saponjski2, H. Huseinagić3, M. Moranjkić4, S. Milošević Medenica5, I. Marinković6, I. Nikolić7, S. Marinkovic8 1Centre for Radiology, Clinic of Neurosurgery, Clinical Centre of Serbia, Belgrade, Serbia 2Clinic of Cardiovascular Surgery, Clinical Centre of Serbia, Belgrade, Serbia 3Department of Radiology, Faculty of Medicine, Kallos University, Tuzla, Bosnia and Herzegovina 4Department of Neurosurgery, Faculty of Medicine, Kallos University, Tuzla, Bosnia and Herzegovina 5Centre for Radiology, Clinical Centre of Serbia, Belgrade, Serbia 6Department of Neurology, Helsinki University Central Hospital, Finland 7Clinic for Neurosurgery, Clinical Centre of Serbia, Belgrade, Serbia 8Institute of Anatomy, Faculty of Medicine, University of Belgrade, Belgrade, Serbia [Received: 8 January 2018; Accepted: 30 January 2018] Background: Identification and anatomic features of the feeding arteries of the arteriovenous malformations (AVMs) is very important due to neurologic, radio- logic, and surgical reasons. Materials and methods: Seventy-seven patients with AVMs were examined by using a digital subtraction angiographic (DSA) and computerised tomographic (CT) examination, including three-dimensional reconstruction of the brain vessels. In addition, the arteries of 4 human brain stems and 8 cerebral hemispheres were microdissected. Results: The anatomic examination showed a sporadic hypoplasia, hyperplasia, early bifurcation and duplication of certain cerebral arteries. The perforating arteries varied from 1 to 8 in number. The features of the leptomeningeal and choroidal vessels were presented.