Vol41n3p131-137
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic: Microsporogenesis and Microgemetogenesis B.Sc. Botany (Hons.) II Paper: IV Group: B Dr
1 Topic: Microsporogenesis and Microgemetogenesis B.Sc. Botany (Hons.) II Paper: IV Group: B Dr. Sanjeev Kumar Vidyarthi Department of Botany Dr. L.K.V.D. College, Tajpur Microsporogenesis and Microgametogenesis Microsporogenesis Microspores i.e., the pollen grains are developed inside microsporangia. The microsporangia are developed inside the corners of the 4-lobed anther. Young anthers are more or less oblong in shape in section and made up of homogeneous mass of meristematic cells without intercellular space with further development, the anther becomes 4-lobed. The outer layer of anther is called epidermis. Below the epidermis, at each corner, some cells become differentiated from others by their dense protoplasm- archesporium or archesporial cells. Each archesporial cell then divides mitotically and forms an outer primary parietal cell and an inner primary sporogenous cell. The outer primary parietal cells form primary parietal cell layer at each corner. Below the parietal cell layer, the primary sporogenous cells remain in groups i.e., the sporogenous tissue. The cells of primary parietal layer then divide both periclinally and anticlinally and form multilayered antheridial wall. The innermost layer of antheridial wall, which remains in close contact with the sporogenous tissue, functions as nutritive layer, called tapetum. The primary sporogenous cells either directly function as spore mother cells or divide mitotically into a number of cells which function as spore mother cells. The spore mother cell undergoes meiotic division and gives rise to 4 microspores arranged tetrahedrally. Structure of Microspores Dr. Sanjeev Kumar Vidyarthi, Dept. of Botany, Dr. L.K.V.D. College, Tajpur 2 Microspore i.e., the pollen grain is the first cell of the male gametophyte, which contains only one haploid nucleus. -

Country Compass 41
COUNTRY COMPASS 41 NWFPs: present and future activities Potential Ongoing activities of government Activities needed NWFPs and non-government agencies Fruit and The World Bank’s Poverty and Health Production of quality fruit and timber Development Profile (PHDP) and the Ministry timber seedlings for better trees of Agriculture, United States Agency for utilization as food, fuelwood, International Development (USAID), German fodder, wood, etc. for local Technical Cooperation (GTZ) and the Bangladesh consumption and to reduce Rural Advancement Committee (BRAC) are forest depletion implementing a project for quality seedling production with community people, local entrepreneurs and farmers’ associations Medicinal No detailed information on medicinal plants Explore medicinal plant plants was found but rural people are using herbal potential throughout the country medicines at large and vendors of herbal as the rural population is medicines exist throughout the country dependent on herbal medicines AFGHANISTAN % Dried Local entrepreneurs produce export quality dried Best-quality fruit fruits figs, black and green raisins, dried apricots production and processing A land of non-wood forest products and nuts and pistachios in different parts of the country of dried fruits and nuts Afghanistan is an exquisitely beautiful for local consumption and export to the Islamic could constitute important country comprised of mountains, scattered Republic of Iran and neighbouring countries export-oriented NWFPs forests and lakes, located in the Hindu Saffron -

Extrapolating Demography with Climate, Proximity and Phylogeny: Approach with Caution
! ∀#∀#∃ %& ∋(∀∀!∃ ∀)∗+∋ ,+−, ./ ∃ ∋∃ 0∋∀ /∋0 0 ∃0 . ∃0 1##23%−34 ∃−5 6 Extrapolating demography with climate, proximity and phylogeny: approach with caution Shaun R. Coutts1,2,3, Roberto Salguero-Gómez1,2,3,4, Anna M. Csergő3, Yvonne M. Buckley1,3 October 31, 2016 1. School of Biological Sciences. Centre for Biodiversity and Conservation Science. The University of Queensland, St Lucia, QLD 4072, Australia. 2. Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield, UK. 3. School of Natural Sciences, Zoology, Trinity College Dublin, Dublin 2, Ireland. 4. Evolutionary Demography Laboratory. Max Planck Institute for Demographic Research. Rostock, DE-18057, Germany. Keywords: COMPADRE Plant Matrix Database, comparative demography, damping ratio, elasticity, matrix population model, phylogenetic analysis, population growth rate (λ), spatially lagged models Author statement: SRC developed the initial concept, performed the statistical analysis and wrote the first draft of the manuscript. RSG helped develop the initial concept, provided code for deriving de- mographic metrics and phylogenetic analysis, and provided the matrix selection criteria. YMB helped develop the initial concept and advised on analysis. All authors made substantial contributions to editing the manuscript and further refining ideas and interpretations. 1 Distance and ancestry predict demography 2 ABSTRACT Plant population responses are key to understanding the effects of threats such as climate change and invasions. However, we lack demographic data for most species, and the data we have are often geographically aggregated. We determined to what extent existing data can be extrapolated to predict pop- ulation performance across larger sets of species and spatial areas. We used 550 matrix models, across 210 species, sourced from the COMPADRE Plant Matrix Database, to model how climate, geographic proximity and phylogeny predicted population performance. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

A Victorian Palm Court
........................................................ ........................................................ A VICTORIAN PALM COURT (An Interpretative Brochure for The New York Botanical Garden) ........................................................ ........................................................ A VICTORIAN PALM COURT (An Interpretative Brochure for The New York Botanical Garden) and PALM SURVIVAL IN A TOUGH WORLD MAUREEN LYNN MURPHY August, 1986 The following manuscripts are submitted as a non-thesis option as partial fulfillment of the requirements for the degree of Master of Science in Ornamental Horticulture. ACKNOWLEDGMENTS I wish to express my sincere appreciation to many people for their help in preparing these manuscripts: The Longwood Gardens Foundation, who provided the generous grant which made my work possible; my thesis committee, Dr. Sherry Kitto, Dr. David Frey, and Dr. Donald Huttletson for their valuable questions, comments, and edits; my thesis committee chairman, and cbordinator of the Longwood Program, Dr. James Swasey for his guidance, assistance, and attention to detail; to Dr. Michael Balick and Mr. Bruce Riggs of The New York Botanical Garden for their advice and suggestions; and to Ms. Dorry Ross, for her skillful editing and gentle manner. A very special thanks goes to Thomas Adarns, not only for his beautiful illustrations, but for his constant encouragement and moral support throughout these past two years. A VICTORIAN PALM COURT INTRODUCTION Palms comprise a very useful plant family, second only in economic importance to the grasses which supply us with wheat, rice, barley, oats, and other grains. Palms provide the world with food (dates, coconuts, palm oil, hearts of palm), beverages (coconut milk, palm wine), clothing (raincoats, hats), medicines (betel nut), construction materials (thatching, irrigation pipes, logs), rope, fiber, carnauba wax, and hundreds of other products. -

Chamaedorea Elegans1
Fact Sheet FPS-119 October, 1999 Chamaedorea elegans1 Edward F. Gilman2 Introduction This palm, native of the dense rain forests of Mexico and Guatemala, is one of the best indoor Chamaedoreas, tolerating crowded roots and low light levels (Fig. 1). Since lower leaves drop from the plant as it grows, older palms have all their foliage on top of the bright green, shiny stem. It grows five to eight feet tall but is usually kept smaller by pruning the stem back nearly to the ground. Growing very slowly, this pale green, single-stemmed palm is most effective when potted three or more to a container. It can also be an effective accent plant in a ground hugging ground cover in a small scale garden. While excellent when used for a house plant, Parlor Palm can also be used outdoors in a shady understory setting as an accent. The showy stems are bright green. General Information Scientific name: Chamaedorea elegans Pronunciation: kam-ee-DOR-ee-uh EL-uh-ganz Common name(s): Parlor Palm, Neanthebella Family: Arecacea Figure 1. Parlor Palm. Plant type: palm USDA hardiness zones: 10B through 11 (Fig. 2) Planting month for zone 10 and 11: year round Description Origin: native to North America Height: 4 to 8 feet Uses: container or above-ground planter; suitable for growing Spread: 2 to 3 feet indoors; border; accent Plant habit: palm; upright Availablity: somewhat available, may have to go out of the Plant density: open region to find the plant Growth rate: slow Texture: medium 1.This document is Fact Sheet FPS-119, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

The Cultivation and Management of Chamaedorea Palms in The
THE CULTIVATION AND MANAGEMENT OF CHAMAEDOREA PALMS IN THE UNDERSTORY OF A TROPICAL RAIN FOREST IN MEXICO A THESIS SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI'I IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BOTANICAL SCIENCES (Botany-Ecology, Evolution, and Conservation Biology) AUGUST 2004 By Parker Clayton Trauemicht Thesis Committee: Tamara Ticktin, Chairperson Donald R. Drake Travis Idol ACKNOWLEDGMENTS As for inspiration, the foundation ofwho I am and my love for science, nature and life - all credit goes to Rindy Trauernicht. The first set ofthanks goes to my family, who has so often provided the means and the encouragement to pursue the many opportunities that have presented themselves over the course ofmy life so far. Many thanks also to my friends here in Hawai'i and elsewhere for their support and healthy amount ofdistraction. I must thank my advisor, Tamara Ticktin, for tracking me down to let me know that she wanted me to be her student. I am truly grateful for her invaluable contribution to my education as both teacher and friend. The other members ofmy committee, Don Drake and Travis Idol, provided helpful criticism and feedback concerning methodology, analyses and the eventual write-up ofthis thesis. Thanks also to Mark Merlin for throwing around ideas. Also, mahalo to Holli and Gerry in the Botany office-the entire department would fall apart without them. Thanks to all ofthe people who helped me in Mexico. First, gracias to the folks at El Proyecto Sierra de Santa Marta in Xalapa, Veracruz. -

Anther Institute of Lifelong Learning, University of Delhi Lesson
Anther Lesson: Anther Author Name: Dr. Bharti Chaudhry and Dr. Anjana Rustagi College/ Department: Ramjas College, Gargi College, University of Delhi Institute of Lifelong Learning, University of Delhi Anther Table of contents Chapter: Anther • Introduction • Structure • Development of Anther and Pollen • Anther wall o Epidermis o Endothecium o Middle layers o Tapetum o Amoeboid Tapetum o Secretory Tapetum o Orbicules o Functions of Orbicules o Tapetal Membrane o Functions of Tapetum • Summary • Practice Questions • Glossary • Suggested Reading Introduction Stamens are the male reproductive organs of flowering plants. They consist of an anther, the site of pollen development and dispersal. The anther is borne on a stalk- like filament that transmits water and nutrients to the anther and also positions it to aid pollen dispersal. The anther dehisces at maturity in most of the angiosperms by a longitudinal slit, the stomium to release the pollen grains. The pollen grains represent the highly reduced male gametophytes of flowering plants that are formed within the sporophytic tissues of the anther. These microgametophytes or 1 Institute of Lifelong Learning, University of Delhi Anther pollen grains are the carriers of male gametes or sperm cells that play a central role in plant reproduction during the process of double fertilization. Figure 1. Diagram to show parts of a flower of an angiosperm Source: http://upload.wikimedia.org/wikipedia/commons/thumb/7/7f/Mature_flower_diagra m.svg/2000px-Mature_flower_diagram.svg.png Figure 2 2 Institute of Lifelong Learning, University of Delhi Anther a. Hibiscus flower; b. Hibiscus stamens showing monothecous anthers; c. Lilium flower showing dithecous anthers Source: a. -

Microsporogenesis and Male Gametogenesis in Jatropha Curcas L. (Euphorbiaceae)1 Huanfang F
Journal of the Torrey Botanical Society 134(3), 2007, pp. 335–343 Microsporogenesis and male gametogenesis in Jatropha curcas L. (Euphorbiaceae)1 Huanfang F. Liu South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China, and Graduate School of Chinese Academy of Sciences, Beijing, 100039, China Bruce K. Kirchoff University of North Carolina at Greensboro, Department of Biology, 312 Eberhart, P.O. Box 26170, Greensboro, NC 27402-6170 Guojiang J. Wu and Jingping P. Liao2 South China Botanical Garden, Chinese Academy of Sciences, Key Laboratory of Digital Botanical Garden in Guangdong, Guangzhou, 510650, China LIU, H. F. (South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, 510650, China, and Graduate School of Chinese Academy of Sciences, Beijing, 100039, China), B. K. KIRCHOFF (University of North Carolina at Greensboro, Department of Biology, 312 Eberhart, P.O. Box 26170, Greensboro, NC 27402-6170), G. J. WU, AND J. P. LIAO (South China Botanical Garden, Chinese Academy of Sciences, Key Laboratory of Digital Botanical Garden in Guangdong, Guangzhou, 510650, China). Microsporogenesis and male gametogenesis in Jatropha curcas L. (Euphorbiaceae). J. Torrey Bot. Soc. 134: 335–343. 2007.— Microsporogenesis and male gametogenesis of Jatropha curcas L. (Euphorbiaceae) was studied in order to provide additional data on this poorly studied family. Male flowers of J. curcas have ten stamens, which each bear four microsporangia. The development of the anther wall is of the dicotyledonous type, and is composed of an epidermis, endothecium, middle layer(s) and glandular tapetum. The cytokinesis following meiosis is simultaneous, producing tetrahedral tetrads. Mature pollen grains are two-celled at anthesis, with a spindle shaped generative cell. -

The SPOROCYTELESS Gene of Arabidopsis Is Required for Initiation of Sporogenesis and Encodes a Novel Nuclear Protein
Downloaded from genesdev.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein Wei-Cai Yang,1 De Ye,1 Jian Xu, and Venkatesan Sundaresan2 The Institute of Molecular Agrobiology, National University of Singapore, Singapore 117604 The formation of haploid spores marks the initiation of the gametophytic phase of the life cycle of all vascular plants ranging from ferns to angiosperms. In angiosperms, this process is initiated by the differentiation of a subset of floral cells into sporocytes, which then undergo meiotic divisions to form microspores and megaspores. Currently, there is little information available regarding the genes and proteins that regulate this key step in plant reproduction. We report here the identification of a mutation, SPOROCYTELESS (SPL), which blocks sporocyte formation in Arabidopsis thaliana. Analysis of the SPL mutation suggests that development of the anther walls and the tapetum and microsporocyte formation are tightly coupled, and that nucellar development may be dependent on megasporocyte formation. Molecular cloning of the SPL gene showed that it encodes a novel nuclear protein related to MADS box transcription factors and that it is expressed during microsporogenesis and megasporogenesis. These data suggest that the SPL gene product is a transcriptional regulator of sporocyte development in Arabidopsis. [Key Words: Arabidopsis mutant; sporogenesis; sporocyte; SPL; nuclear protein] Received May 12, 1999; revised version accepted July 1, 1999. The life cycle of plants consists of an alternation be- 1994), although several sporophytic mutants that affect tween a diploid, sporophytic generation and a haploid, sporogenesis have been reported (Robinson-Beers et al. -
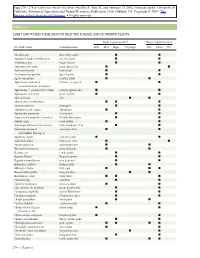
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum -

II (Hons.&Subs.) Paper: Ivth Topic: Microgametogenesis Lecture
Name: Dr. Rachana Shalini Subject: Botany Class: Deg.-II (Hons.&Subs.) Paper: IVth Topic: Microgametogenesis Lecture no. 18 MICROGAMETOGENESIS: Microgametogenesis is a process by which progressive development of the unicellular microspores takes place where they get developed to mature microgametophytes containing gametes. The development phase of microspores takes place with the onset of expansion of microspore. In this phase, a single large vacuole is produced within the microspore cell. The formation of the vacuole results in the movement of the nucleus of the microspore to an eccentric position. The displacement of the nucleus occurs against the wall of the microspore cell. At this position within the cell, the nucleus undergoes mitosis. Microspore .i.e, the pollen grain is the first cell of the male gametophyte, which posses one haploid nucleus. During early stages of development, it remains within the microsporangium i.e, its germination starts within the microsporangium. The nucleus of the pollen grain undergoes unequal division and forms a large vegetative or tube cell and a small generative cell. Initially, the generative cell remains lying at one corner of the spore wall. Later it gets detached and gets suspended in the cytoplasm of the vegetative cell (forms a 2 celled stage consisting of vegetative cell and generative cell). Later on the generative cell divides and give rise to two cells that are the male gametes (forms 3 celled stage consisting of two male gametes and the vegetative cell) The process of microgametogenesis ends here and later fertilisation occurs. The division of the generative cell may either take place in the pollen grain or in the newly formed pollen tube) The nucleus of the vegetative cell is known as the tube nucleus.