The Cultivation and Management of Chamaedorea Palms in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hybrids in Chamaedorea D,Q,Vdbannv, Jn
19671 BARRY:HYBRIDS r21 Endemic to Cuba, where found on longer and stouter prickles, the upper calcareous soils in all the provinces margins fibrous and extending along according to Le6n, Flora d,e Cuba I: the petiole nearly to the first pinnae, 245. 1946. the free portion of the petiole about 45 cm. (18 in.) long with brown or yel- cRISpA(Humboldt, Bon- Glsrnococos lowish prickles, some very stoutI ra- E. Moore, lr. pland & Kunth) H. chis more than 2.5 m. (87/z ft.) long, noa. often with short yellowish prickles along Cocos crispa Humboldt, BonPland & the lower margin and on the lower sur- Kunth, Nova Genera et SPecies face near the junction with the petiole; Plantarum 1:302 f{olio 242]- pinnae to 120 or more on each side of 1816. the rachis, the lower onesshort, narrow, Acrocornia crispo (Humboldt, Bon- and closely placed, those in the center pland & Kunth) C. F. Baker ex to f m. (3r/2fi.) Iong, 3 cm. (1 3/16 Beccari, in Pomona College Jour' itt.) wide, the midnerve gr€en, the nal of Economic Botany 2: 364. lower surface pale. Inflorescenceto 1.5 I9T2. m. (5 ft.) long or more, the upper bract Gastrococosarrnentalis S. A. Morales, brown tomentosel peduncle about 7'5 in Repertorio Fisico-Natural de la dm. (.27/z ft.) long, densely brown Isla de Cuba I: 58. 1865. or yellowish prickly; rachis about as Acrocomia armentalis (S. A. Mora- long as peduncle,smooth or with prick' les) L. H. Bailey, Hortus Second les at base; rachillaeto 30 cm. -

Country Compass 41
COUNTRY COMPASS 41 NWFPs: present and future activities Potential Ongoing activities of government Activities needed NWFPs and non-government agencies Fruit and The World Bank’s Poverty and Health Production of quality fruit and timber Development Profile (PHDP) and the Ministry timber seedlings for better trees of Agriculture, United States Agency for utilization as food, fuelwood, International Development (USAID), German fodder, wood, etc. for local Technical Cooperation (GTZ) and the Bangladesh consumption and to reduce Rural Advancement Committee (BRAC) are forest depletion implementing a project for quality seedling production with community people, local entrepreneurs and farmers’ associations Medicinal No detailed information on medicinal plants Explore medicinal plant plants was found but rural people are using herbal potential throughout the country medicines at large and vendors of herbal as the rural population is medicines exist throughout the country dependent on herbal medicines AFGHANISTAN % Dried Local entrepreneurs produce export quality dried Best-quality fruit fruits figs, black and green raisins, dried apricots production and processing A land of non-wood forest products and nuts and pistachios in different parts of the country of dried fruits and nuts Afghanistan is an exquisitely beautiful for local consumption and export to the Islamic could constitute important country comprised of mountains, scattered Republic of Iran and neighbouring countries export-oriented NWFPs forests and lakes, located in the Hindu Saffron -

Arizona Landscape Palms
Cooperative Extension ARIZONA LANDSCAPE PALMS ELIZABETH D AVISON Department of Plant Sciences JOHN BEGEMAN Pima County Cooperative Extension AZ1021 • 12/2000 Issued in furtherance of Cooperative Extension work acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, James A. Christenson, Director, Cooperative Extension, College of Agriculture and Life Sciences, The University of Arizona. The University of Arizona College of Agriculture and Life Sciences is an equal opportunity employer authorized to provide research, educational information and other services to individuals and institutions that function without regard to sex, race, religion, color, national origin, age, Vietnam Era Veteran's status, or disability. Contents Landscape Use ......................................... 3 Adaptation ................................................ 3 Planting Palms ......................................... 3 Care of Established Palms...................... 5 Diseases and Insect Pests ....................... 6 Palms for Arizona .................................... 6 Feather Palms ........................................... 8 Fan Palms................................................ 12 Palm-like Plants ..................................... 16 This information has been reviewed by university faculty. ag.arizona.edu/pubs/garden/az1121.pdf 2 The luxuriant tropical appearance and stately Adaptation silhouette of palms add much to the Arizona landscape. Palms generally can be grown below the 4000 ft level Few other plants are as striking in low and mid elevation in Arizona. However, microclimate may make the gardens. Although winter frosts and low humidity limit difference between success and failure in a given location. the choices somewhat, a good number of palms are Frost pockets, where nighttime cold air tends to collect, available, ranging from the dwarf Mediterranean Fan should be avoided, especially for the tender species. Palms palm to the massive Canary Island Date palm. -

Extrapolating Demography with Climate, Proximity and Phylogeny: Approach with Caution
! ∀#∀#∃ %& ∋(∀∀!∃ ∀)∗+∋ ,+−, ./ ∃ ∋∃ 0∋∀ /∋0 0 ∃0 . ∃0 1##23%−34 ∃−5 6 Extrapolating demography with climate, proximity and phylogeny: approach with caution Shaun R. Coutts1,2,3, Roberto Salguero-Gómez1,2,3,4, Anna M. Csergő3, Yvonne M. Buckley1,3 October 31, 2016 1. School of Biological Sciences. Centre for Biodiversity and Conservation Science. The University of Queensland, St Lucia, QLD 4072, Australia. 2. Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield, UK. 3. School of Natural Sciences, Zoology, Trinity College Dublin, Dublin 2, Ireland. 4. Evolutionary Demography Laboratory. Max Planck Institute for Demographic Research. Rostock, DE-18057, Germany. Keywords: COMPADRE Plant Matrix Database, comparative demography, damping ratio, elasticity, matrix population model, phylogenetic analysis, population growth rate (λ), spatially lagged models Author statement: SRC developed the initial concept, performed the statistical analysis and wrote the first draft of the manuscript. RSG helped develop the initial concept, provided code for deriving de- mographic metrics and phylogenetic analysis, and provided the matrix selection criteria. YMB helped develop the initial concept and advised on analysis. All authors made substantial contributions to editing the manuscript and further refining ideas and interpretations. 1 Distance and ancestry predict demography 2 ABSTRACT Plant population responses are key to understanding the effects of threats such as climate change and invasions. However, we lack demographic data for most species, and the data we have are often geographically aggregated. We determined to what extent existing data can be extrapolated to predict pop- ulation performance across larger sets of species and spatial areas. We used 550 matrix models, across 210 species, sourced from the COMPADRE Plant Matrix Database, to model how climate, geographic proximity and phylogeny predicted population performance. -

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Ecological Aspects of the Interaction Between Chamaedorea Tepejilote, A
B6 PRINCIPES [Vor. 35 Principes,35(2), 1991, pp. 86 93 EcologicalAspects of the Interaction between Chamaedoreatepejilote, a DioeciousPalm and Calyptocephalamarginipennis, a Herbivorous Beetle, in a Mexican Rain Forest K. Oveue.* ANDR. DIRZo Centro d.eEcol.osia, WAM, Apartado Postal 7O-275, M6xico, 04510, D.F. Assrnecr ers have made studies regarding intraspe- cific differencesin herbivory in the context Liebm. ex Mart., an Chamaedorea tepejilote of polymorphic plant systemsin temperate abundant dioeciouspalm in the lowland rain forest of Los Tuxtlas (SoutheastMexico), typically bears evi- zones(e.g., Jones 1966, Cates1975, Dirzo dence of leaf damage by Calyptocephala margini- and Harper I982a). Dioecy constitutes pennis Bohem. (Chrysomelidae).The beetles mark- one of the most common polymorphic sys- edly prefer this palm in comparison to other plants tems, particularly in some Neotropical on this site, including other sympatric speciesin the same genus. Additionally, beetlesshowed some mar- communities(Croat 1979, Bawa and Opler ginal preference for the foliage of female plants under 1975, Bawa 1980, Flores and Schemske experimental and field conditions. Sex-related phy- 1984, Bawa et al. 1985). Suggestionin tochemical attributes of the palm, such as secondary the literature of sex-baseddifferences in and nutritional characteristicsof the foli- comoounds herbivory include male inflorescencessuf- age, did not differ markedly between sexes and did not seemto be the proximal causesof the inter-sexual fering higher levels of predation than female differences in herbivory. Thus, the hypothesis that inflorescences(Fryxell and Lulcefahr1967, differencesin resourceallocation to defenseand nutri Bawa and Opler 1978) and casesin which tional quality, resulting from sex-related differential herbivores preferentially feed either on allocation to reproduction, is not supported by this study. -

A Victorian Palm Court
........................................................ ........................................................ A VICTORIAN PALM COURT (An Interpretative Brochure for The New York Botanical Garden) ........................................................ ........................................................ A VICTORIAN PALM COURT (An Interpretative Brochure for The New York Botanical Garden) and PALM SURVIVAL IN A TOUGH WORLD MAUREEN LYNN MURPHY August, 1986 The following manuscripts are submitted as a non-thesis option as partial fulfillment of the requirements for the degree of Master of Science in Ornamental Horticulture. ACKNOWLEDGMENTS I wish to express my sincere appreciation to many people for their help in preparing these manuscripts: The Longwood Gardens Foundation, who provided the generous grant which made my work possible; my thesis committee, Dr. Sherry Kitto, Dr. David Frey, and Dr. Donald Huttletson for their valuable questions, comments, and edits; my thesis committee chairman, and cbordinator of the Longwood Program, Dr. James Swasey for his guidance, assistance, and attention to detail; to Dr. Michael Balick and Mr. Bruce Riggs of The New York Botanical Garden for their advice and suggestions; and to Ms. Dorry Ross, for her skillful editing and gentle manner. A very special thanks goes to Thomas Adarns, not only for his beautiful illustrations, but for his constant encouragement and moral support throughout these past two years. A VICTORIAN PALM COURT INTRODUCTION Palms comprise a very useful plant family, second only in economic importance to the grasses which supply us with wheat, rice, barley, oats, and other grains. Palms provide the world with food (dates, coconuts, palm oil, hearts of palm), beverages (coconut milk, palm wine), clothing (raincoats, hats), medicines (betel nut), construction materials (thatching, irrigation pipes, logs), rope, fiber, carnauba wax, and hundreds of other products. -

Chamaedorea Elegans1
Fact Sheet FPS-119 October, 1999 Chamaedorea elegans1 Edward F. Gilman2 Introduction This palm, native of the dense rain forests of Mexico and Guatemala, is one of the best indoor Chamaedoreas, tolerating crowded roots and low light levels (Fig. 1). Since lower leaves drop from the plant as it grows, older palms have all their foliage on top of the bright green, shiny stem. It grows five to eight feet tall but is usually kept smaller by pruning the stem back nearly to the ground. Growing very slowly, this pale green, single-stemmed palm is most effective when potted three or more to a container. It can also be an effective accent plant in a ground hugging ground cover in a small scale garden. While excellent when used for a house plant, Parlor Palm can also be used outdoors in a shady understory setting as an accent. The showy stems are bright green. General Information Scientific name: Chamaedorea elegans Pronunciation: kam-ee-DOR-ee-uh EL-uh-ganz Common name(s): Parlor Palm, Neanthebella Family: Arecacea Figure 1. Parlor Palm. Plant type: palm USDA hardiness zones: 10B through 11 (Fig. 2) Planting month for zone 10 and 11: year round Description Origin: native to North America Height: 4 to 8 feet Uses: container or above-ground planter; suitable for growing Spread: 2 to 3 feet indoors; border; accent Plant habit: palm; upright Availablity: somewhat available, may have to go out of the Plant density: open region to find the plant Growth rate: slow Texture: medium 1.This document is Fact Sheet FPS-119, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -

Coleoptera: Chrysomelidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Center for Systematic Entomology, Gainesville, Insecta Mundi Florida 9-2-2011 Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) R. F. C. Naczi The New York Botanical Garden, [email protected] C. L. Staines National Museum of Natural History, Smithsonian Institution, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/insectamundi Part of the Entomology Commons Naczi, R. F. C. and Staines, C. L., "Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae)" (2011). Insecta Mundi. 702. https://digitalcommons.unl.edu/insectamundi/702 This Article is brought to you for free and open access by the Center for Systematic Entomology, Gainesville, Florida at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Insecta Mundi by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. INSECTA MUNDI A Journal of World Insect Systematics 0190 Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) R. F. C. Naczi The New York Botanical Garden 2900 Southern Blvd. Bronx, NY 10458-5126, U.S.A. C. L. Staines Department of Entomology, MRC 187 National Museum of Natural History, Smithsonian Institution Washington, DC 20013-7012, U.S.A. Date of Issue: September 2, 2011 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL R. F. C. Naczi and C. L. Staines Noteworthy Records of Hispines from Belize (Coleoptera: Chrysomelidae) Insecta Mundi 0190: 1-6 Published in 2011 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 U. S. A. http://www.centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. -

Population Structure of the Dominant Palm Species in the Understory of a Mexican Lowland Rain Forest
TROPICS Vol. 2 (1): 23 - 28 Issued August, 1992 Population Structure of the Dominant Palm Species in the Understory of a Mexican Lowland Rain Forest Ken OYAMA Centro de Ecologia, U.N.A.M. Apartado Postal 70-275, Mexico, 04510, D.E Current address: Department of Biology, University of Tokyo, Komaba 3-8-1, Meguro-ku, Tokyo 153, Japan Rodolfo DIRZO Centro de Ecologia, U.N. A.M. Apartado Postal 70-275, Mexico, 04510, D.E Guillermo IBARRA-MANRIQUEZ Estaci6n de Biologia Tropical Los Tuxtlas, Apartado Postal 94, Los Tuxtlas, Veracruz, Mexico Abstract Population structure was analyzed for five of the most common species of palms, Astrocaryum mexicanum, Bactris tricophylla, Chamaedorea oblongata, C. tepejilote and Geonoma oxycarpa in a Mexican lowland rain forest. Height and cover of palms with a height> 1.5 m were measured in three 600 m2 plots. The most common species in terms of density were C. tepejilote and A. mexicanum. The highest accumulated cover corresponded to A. mexicanum.Taller palms of C. tepejilote showed higher cover. Almost all palm species had the same architectural model (Comer) and B. tricophylla had the Tomlinson model. Richness of palm species was low compared to other tropical forests although the understory palms in this community represent more than 50 % of all the individuals of the understory vegetation at Los Tuxtlas. The genus Chamaedorea was the most diversified (5 species) while the other genera had only one species. Key words: Mexico / Neotropics / palms / population structure / species diversity / rain forest Few studies have been carried out on the diversity and structure of the palm components within tropical forest communities, although palms usually occur in high numbers in mixed tropical forests. -

A Comparison of Hispine Beetles (Coleoptera: Chrysomelidae) Associated with Three Orders of Monocot Host Plants in Lowland Panama
International Journal of Tropical Insect Science Vol. 27, No. 3/4, pp. 159-171, 2008 DOI: 10.1017/S1742758407864071 © icipe 2008 A comparison of hispine beetles (Coleoptera: Chrysomelidae) associated with three orders of monocot host plants in lowland Panama Christophe Meskens1*, Donald Windsor2 and Thierry Hance1 1 Unite d'Ecologie et de Biogeographie, Biodiversity Research Centre, Universite catholique de Louvain, 4-5, Place Croix du Sud, Louvain-la- Neuve 1348, Belgium: ^Smithsonian Tropical Research Institute, Apartado 0843-03092, Panama (Accepted 17 October 2007) Abstract. The feeding traces in fossil ginger leaves and the conserved phylogenetic relationships seen today in certain clades of hispine beetles on their monocot hosts point towards a long and intimate plant-insect evolutionary relationship. Studies in the 1970s and 1980s documented the rich fauna of rolled-leaf hispine beetles and their association with the Neotropical monocot family Heliconiaceae in Central America. In this report, the taxonomic breadth of these early studies is expanded to include species in the families, Marantaceae, Poaceae, Arecaceae and Costaceae, all with species occurring sympatrically with the Heliconiaceae in lowland Panama. Additionally, the analysis is widened to include open-leaf scraping and internal leaf-mining clades of hispoid Cassidinae. The censuses add more than 5080 Cassidinae herbivore occurrence records on both open and unfurled new leaf rolls of 4600 individual plants. Cluster analysis reveals that while many Hispinae species tend to group with plant species in only one of the three monocot orders, 9 of 16 Hispinae species on Zingiberales hosts were recorded in substantial numbers on both the Heliconiaceae and the Marantaceae, indicating an underlying pattern of feeding flexibility at the host plant family level. -
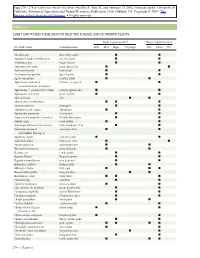
Light and Water Guidelines for Selected Foliage and Flowering Plants
Table 11.1 LIGHT AND WATER GUIDELINES FOR SELECTED FOLIAGE AND FLOWERING PLANTS Light requirements* Water requirements† Scientific name Common name Low Med High Very high Dry Moist Wet Abutilon spp. flowering maple II Acalypha hispida (A.wilkesiana) chenille plant I I Achimenes spp. magic flower II Adiantum cuneatum maidenhair fern II Aechmea fasciata bromeliad II Aeschynanthus pulcher lipstick plant II Agave americana century plant II Aglaonema modestum Chinese evergreen II (A.commutatum,A.simplex) Aglaonema ϫ pseudo-bracteatum golden aglaonema II Aglaonema roebelenii pewter plant II Aloe variegata aloe II Alternanthera bettzickiana II I Ananas comosus pineapple I I Anthurium andreanum anthurium II Aphelandra squarrosa zebra plant II Araucaria heterophylla (A.excelsa) Norfolk Island pine II Ardisia crispa coral ardisia II Asparagus plumosus (A.setaceus) bride’s bouquet fern II Asparagus sprengeri asparagus fern II (A.densiflora Sprenger) Aspidistra elatior cast-iron plant I I Asplenium nidus bird’s nest fern I I Aucuba japonica gold-dust plant I I Beaucarnea recurvata pony tail palm I I Begonia rex rex begonia I I Begonia ‘Rieger’ Rieger begonia I I Begonia semperflorens wax begonia II Beloperone guttata shrimp plant II Billbergia zebrina billbergia III Bougainvillea glabra bougainvillea II Browallia speciosa bush violet II I Caladium spp. caladium II Calathea makoyana peacock plant II Calceolaria herbeahybrida pocketbook plant II Campanula isophylla star-of-Bethlehem II Capsicum annuum Christmas pepper II Carissa grandiflora Natal plum