How Baleen Whales Feed: the Biomechanics of Engulfment and Filtration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toothed Vs. Baleen Whales Monday
SPOT THE DIFFERENCE: TOOTHED VS. BALEEN WHALES MONDAY Their classifications help to give you the answer, so what do you think the most obvious difference is in a toothed whale versus a baleen whale? Your clues are in the close-up photos, below! PHOTO: TASLI SHAW PHOTO: CINDY HANSEN Answer: The most obvious difference between a toothed whale and a baleen whale is the way that they feed and what’s inside their mouth. Toothed whales (including all dolphins and porpoises) have teeth, like we do, and they actively hunt fish, squid, and other sea creatures. Their teeth help them capture, bite, and tear their food into smaller pieces before swallowing. Baleen whales have several hundred plates that hang from their upper jaw, instead of teeth. These plates are made of keratin, the same substance as our hair and fingernails, and are used to filter food from the water or the sediment. Once the food has been trapped in the baleen plates, the whales will use their massive tongues to scrape the food off and swallow it. SPOT THE DIFFERENCE: TOOTHED VS. BALEEN WHALES TUESDAY The photos provided show specific prey types for resident orcas and for the gray whales that stop to feed in Saratoga Passage in the spring. Besides being two different species, what is another difference between these prey types? Who eats what and what makes you think that? Answer: The photos show Chinook salmon and ghost shrimp. Other than being two different species, their main difference is size! A toothed whale, like a resident orca, uses their teeth to capture, bite, and tear Chinook salmon into smaller pieces to be shared with other orcas in their family. -

Balaenoptera Bonaerensis – Antarctic Minke Whale
Balaenoptera bonaerensis – Antarctic Minke Whale compared to B. bonaerensis. This smaller form, termed the “Dwarf” Minke Whale, may be genetically different from B. bonaerensis, and more closely related to the North Pacific Minke Whales, and thus has been classified B. acutorostrata (Wada et al. 1991; IWC 2001). This taxonomic position, although somewhat controversial, has been accepted by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and the Convention on Migratory Species (CMS). Assessment Rationale The current IWC global estimate of abundance of Antarctic Dr. Meike Scheidat Minke Whales is about 500,000 individuals. The abundance estimates declined from about 700,000 for the second circumpolar set of abundance survey cruises Regional Red List status (2016) Least Concern* (1985/86 to 1990/91) to about 500,000 for the third National Red List status (2004) Least Concern (1991/92 to 2003/04). Although this decline was not statistically significant, the IWC Scientific Committee does Reasons for change No change consider these results to reflect a change. However, Global Red List status (2008) Data Deficient whether this change is genuine or attributed to greater proportions of pack ice limiting the survey extent, has not TOPS listing (NEMBA) (2007) None yet been determined. More detailed results from an CITES listing (1986) Appendix I assessment model are available for the mid-Indian to the mid-Pacific region, and suggest that the population Endemic No increased to a peak in 1970 and then declined, with it *Watch-list Data being unclear whether this decline has levelled off or is still continuing past 2000. -

Lunge Filter Feeding Biomechanics Constrain Rorqual Foraging Ecology Across Scale S
© 2020. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2020) 223, jeb224196. doi:10.1242/jeb.224196 RESEARCH ARTICLE Lunge filter feeding biomechanics constrain rorqual foraging ecology across scale S. R. Kahane-Rapport1,*, M. S. Savoca1, D. E. Cade1,2, P. S. Segre1, K. C. Bierlich3, J. Calambokidis4, J. Dale3, J. A. Fahlbusch1, A. S. Friedlaender2, D. W. Johnston3, A. J. Werth5 and J. A. Goldbogen1 ABSTRACT morphological scaling (Haldane, 1926), resulting in functional Fundamental scaling relationships influence the physiology of vital trade-offs that ultimately impact evolution and ecology. rates, which in turn shape the ecology and evolution of organisms. For The physiological advantages and disadvantages associated with diving mammals, benefits conferred by large body size include different body sizes have wide-ranging effects, from behavior to life reduced transport costs and enhanced breath-holding capacity, history. For example, the smallest animals have the lowest absolute thereby increasing overall foraging efficiency. Rorqual whales feed energetic demands (Kelt and Van Vuren, 1999), yet they may also by engulfing a large mass of prey-laden water at high speed and struggle with thermoregulation and be forced to compensate by filtering it through baleen plates. However, as engulfment capacity increasing their metabolism (Scholander et al., 1950; Taylor et al., increases with body length (engulfment volume∝body length3.57), the 1980). Small size enables high performance maneuverability and surface area of the baleen filter does not increase proportionally agility (Domenici, 2001), but may limit maximum attainable speeds (baleen area∝body length1.82), and thus the filtration time of larger (Carrier, 1994; Hirt et al., 2017). -

Mandible Allometry in Extant and Fossil Balaenopteridae (Cetacea: Mammalia): the Largest Vertebrate Skeletal Element and Its Role in Rorqual Lunge Feeding
bs_bs_banner Biological Journal of the Linnean Society, 2013, 108, 586–599. With 6 figures Mandible allometry in extant and fossil Balaenopteridae (Cetacea: Mammalia): the largest vertebrate skeletal element and its role in rorqual lunge feeding NICHOLAS D. PYENSON1,2*, JEREMY A. GOLDBOGEN3 and ROBERT E. SHADWICK4 1Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, Washington, DC, 20013-7013, USA 2Departments of Mammalogy and Paleontology, Burke Museum of Natural History and Culture, Seattle, WA 98195, USA 3Cascadia Research Collective, 218½ West 4th Avenue, Olympia, WA, USA 4Department of Zoology, University of British Columbia, 6270 University Boulevard, Vancouver, BC, Canada V6T 1Z4 Received 4 July 2012; revised 10 September 2012; accepted for publication 10 September 2012 Rorqual whales (crown Balaenopteridae) are unique among aquatic vertebrates in their ability to lunge feed. During a single lunge, rorquals rapidly engulf a large volume of prey-laden water at high speed, which they then filter to capture suspended prey. Engulfment biomechanics are mostly governed by the coordinated opening and closing of the mandibles at large gape angles, which differentially exposes the floor of the oral cavity to oncoming flow. The mouth area in rorquals is delimited by unfused bony mandibles that form kinetic linkages to each other and with the skull. The relative scale and morphology of these skeletal elements have profound consequences for the energetic efficiency of foraging in these gigantic predators. Here, we performed a morphometric study of rorqual mandibles using a data set derived from a survey of museum specimens. Across adult specimens of extant balaenopterids, mandibles range in size from ~1–6 m in length, and at their upper limit they represent the single largest osteological element of any vertebrate, living or extinct. -

Rorqual Whale (Balaenopteridae) Surface Lunge-Feeding Behaviors: Standardized Classification, Repertoire Diversity, and Evolutionary Analyses
MARINE MAMMAL SCIENCE, 30(4): 1335–1357 (October 2014) © 2014 Society for Marine Mammalogy DOI: 10.1111/mms.12115 Rorqual whale (Balaenopteridae) surface lunge-feeding behaviors: Standardized classification, repertoire diversity, and evolutionary analyses BRIAN W. KOT,1 Department of Ecology and Evolutionary Biology, University of California, 621 Charles E. Young Drive South, Los Angeles, California 90095-1606 U.S.A. and Mingan Island Cetacean Study, Inc., 378 Bord de la Mer, Longue-Pointe-de-Mingan, Quebec G0G 1V0, Canada; RICHARD SEARS, Mingan Island Cetacean Study, Inc., 378 Bord de la Mer, Longue-Pointe-de-Mingan, Quebec G0G 1V0, Canada; DANY ZBINDEN, Meriscope Marine Research Station, 7 chemin de la Marina, Portneuf-sur-Mer, Quebec G0T 1P0, Canada; ELIZABETH BORDA, Department of Marine Biology, Texas A&M University, 200 Seawolf Parkway, Galveston, Texas 77553, U.S.A.; MALCOLM S. GORDON, Department of Ecology and Evolutionary Biology, University of California, 621 Charles E. Young Drive South, Los Angeles, California 90095-1606, U.S.A. Abstract Rorqual whales (Family: Balaenopteridae) are the world’s largest predators and sometimes feed near or at the sea surface on small schooling prey. Most rorquals cap- ture prey using a behavioral process known as lunge-feeding that, when occurring at the surface, often exposes the mouth and head above the water. New technology has recently improved historical misconceptions about the natural variation in rorqual lunge-feeding behavior yet missing from the literature is a dedicated study of the identification, use, and evolution of these behaviors when used to capture prey at the surface. Here we present results from a long-term investigation of three rorqual whale species (minke whale, Balaenoptera acutorostrata; fin whale, B. -

Humpback Whales 101
G3 U5 OVR GRADE 3 UNIT 5 OVERVIEW Humpback Whales 101 Introduction Humpback whales are highly intelligent marine mammals that depend on specifi c environmental conditions to survive. They migrate north to nutrient-rich waters of Alaska to feed during the summer, and south to tropical, but nutrient-poor, warm waters in winter to give birth and mate. Humpback whales feed on huge amounts of small fi sh and plankton that are abundant in northern marine environments in spring and summer. Adult whales maintain a thick layer of insulating blubber under their skin that keeps internal body temperatures constant. Whales are not born with insulating blubber and would freeze in cold Alaskan waters, which may explain whale migration to tropical environments in winter to give birth, and thus perpetuate survival of the species. Brainstorming the amazing adaptations these marine mammals have undergone over millions of years to survive in ocean environments, brings this unit to life for the students. They then imagine body feature changes that would be required for humans to adapt to similar environments. Students also learn that humpback whales, like humans, are warm-blooded, give birth, engage in courtships, mate, nurse their young, and protect them from predators. Students study whale body features and crucial roles they play during migration, feeding, and mating. The unit’s main focus is on whale behavior while in the mating and nursing grounds in Hawai‘i. Like researchers, students follow the scientifi c inquiry process to answer questions. Through hands-on lab activities and fun games that complement lessons, students replicate the feeding behaviors of whales, and create bar graphs comparing the feeding styles of baleen and toothed whales. -
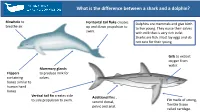
What Is the Difference Between a Shark and a Dolphin?
What is The Difference BetweenWhat a is the difference between a shark and a dolphin? Shark and a Dolphin? Blowhole to Horizontal tail fluke creates Dolphins are mammals and give birth breathe air. up and down propulsion to to live young. They nurse their calves swim. with milk that is very rich in fat. Sharks are fish. Most lay eggs and do not care for their young. Gills to extract oxygen from water. Mammary glands Flippers to produce milk for containing calves. bones similar to human hand bones. Vertical tail fin creates side Additional fins , Fin made of strong, to side propulsion to swim. second dorsal, flexible tissue pelvic and anal. called cartilage. What is The Difference BetweenWhat a is the difference between a whale and a dolphin? Shark and a Dolphin? Two blowholes Baleen Whale Baleen is the bristle like structure to breathe air. in a whale’s upper jaw which it uses to filter small fish or crustaceans from the water. Single blowhole to Many species we call whales breathe air. are more closely related to Throat pleats in some baleen dolphins. Generally scientists whales expand to fill with water Dolphin talk about baleen whales and small fish or crustaceans and and toothed then contract, pushing the water whales. Toothed whales back through the baleen. include sperm whales, beaked whales, and all Teeth on the upper and lower porpoises and dolphins. jaws to grab fish, squid or other Killer whales and pilot prey. They use echolocation to whales are actually dolphins. find their food. What is The Difference BetweenEcholocation a Shark and a Dolphin? Toothed whales (dolphins, porpoises and species like pilot Brain processes Nasal passage whales and killer whales) use echolocation to navigate and find signals to form an contains their food. -

WHALES of the ANTARCTIC PENINSULA Science and Conservation for the 21St Century CONTENTS
THIS REPORT HAS BEEN PRODUCED IN COLLABORATION WITH REPORT ANTARCTICA 2018 WHALES OF THE ANTARCTIC PENINSULA Science and Conservation for the 21st Century CONTENTS Infographic: Whales of the Antarctic Peninsula 4 1. PROTECTING OCEAN GIANTS UNDER INCREASING PRESSURES 6 2. WHALES OF THE ANTARCTIC PENINSULA 8 Species found in the Antarctic Peninsula are still recovering from commercial whaling 10 Infographic: Humpback whale migration occurs over multiple international and national jurisdictions 12 Whales face several risks in the region and during migrations 15 Authors: Dr Ari Friedlaender (UC Santa Cruz), Michelle Modest (UC Baleen whales use the Antarctic Peninsula to feed on krill Santa Cruz) and Chris Johnson (WWF Antarctic programme). – the keystone species of the Antarctic food chain 16 Contributors: Infographic: The Western Antarctic Peninsula is critical Dr David Johnston (Duke University), Dr Jennifer Jackson feeding habitat for humpback whales 20 (British Antarctic Survey) and Dr Sarah Davie (WWF-UK). Whales play a critical role in Southern Ocean ecosystems 22 Acknowledgements: Special thanks to Rod Downie (WWF-UK), Dr Reinier Hille Ris Lambers (WWF-NL), Rick Leck (WWF-Aus), 3. NEW SCIENCE IS CHANGING OUR UNDERSTANDING OF WHALES 24 Duke University Marine Robotics and Remote Sensing Lab, California Ocean Alliance and One Ocean Expeditions. Technology is providing scientists and policymakers with data to better understand, monitor and conserve Antarctic whales 26 Graphic Design: Candy Robertson Copyediting: Melanie Scaife Satellite and suction-cup tags uncover whale foraging areas and behaviour 28 Front cover photo: © Michael S. Nolan / Robert Harding Picture Library / National Geographic Creative / WWF Long-Term Ecological Research – Palmer Station, Antarctica 29 Photos taken under research permits include: Dr Ari Drones uncovering a new view from above 30 Friedlaender NMFS 14809, ACA 2016-024 / 2017-034, UCSC IACUC friea1706, and ACUP 4943. -

Yearly Trend of Blubber Thickness in the Antarctic Minke Whale Balaenoptera Bonaerensis in Areas IV and V
NOT TO BE CITED WITHOUT PERMISSION BY THE AUTHORS JA/J05/JR9 Yearly trend of blubber thickness in the Antarctic minke whale Balaenoptera bonaerensis in Areas IV and V 1) 1) 2) KENJI KONISHI , TSUTOMU TAMURA AND LARS WALLØW 1)The Institute of Cetacean Research, 4-5, Toyomi-cho, Chuo-ku, Tokyo 104-0055, Japan 2)Department of Physiology, University of Oslo, 1103, Blindern N-0317 Oslo, Norway KEYWORDS: ANTARCTIC MINKE WHALE; BODY CONDITION; BALAENOPTERA; BLUBBER THICKNESS; SCIENTIFIC PERMITS ABSTRACT To examine the recent trend of body condition and factors that affect the body condition in Antarctic minke whale, blubber thickness from 1987/88 to 2003/04 in Areas IV and V were analysed. In the stepwise multiple regression analyses, the blubber thickness at mid-lateral position was used as dependent variable, and date, diatom film adhesion, sex, longitude (Area) and year was used as explanatory variables. To see the trend of body condition in the end of the feeding season, blubber thickness in February were also examined in each Area and sex. The results of multiple regressions confirmed that blubber thickness of the Antarctic minke whale has decreased for JARPA survey period, and showed all explanatory variables were detected as predictor variables. The results in February also showed decline of blubber thickness over JARPA survey without in pregnant female in Area V. These results indicated that food availability for Antarctic minke whale in Antarctic has decreased. This decline possibly caused by both intraspecific and interspecies competitions for krill resources. INTRODUCTION The Antarctic Ocean consists of simple food web with high productivity and is an important feeding area for the baleen whale, which is one of the major predator groups, consuming mostly on Antarctic krill (Euphausia superba). -

Humpback Whale Fact Sheet
Learn about: Humpback Whale As Gaeilge: An Míol mór dronnach Scientific name: Megaptera novaeangliae What is it? Whales are marine mammals, they belong to a group called cetaceans (pronounced set-AY- shans), which includes both dolphins and whales. Humpback whales are a large species of baleen whale. They are black in colour with a white throat and belly, and long flippers. They have a large tail with a wavy edge, which they stick up in the air as they dive. Humpbacks don’t actually have a hump on their back, but they do bend their back when diving. Their head is covered with bumps called tubercles. Each bump contains at least one stiff hair, believed to help detect movement in the water. How big is it? Humpback whales are usually about 13m long but can reach up to 16m long. The females are bigger than the males. They weigh up to 36,000kg! Where does it live? Humpback whales are widespread throughout the world’s oceans. In the colder months they stay in warm, tropical waters where they give birth to their calves. In the warmer months they migrate to colder waters further from the equator to feed. The best time to spot humpback whales in Irish water is July and August, where you might spot them off the Cork or Kerry coast. Humpback whale can live to be 50 years old. What does it eat? Humpbacks are filter feeders, feeding on plankton, krill (shrimp) and small fish like herring and sprat. For this reason, they don’t have teeth, instead they have baleen. -

Kawamura, A. a Review of Food of Balaenopterid Whales. 155-197
A REVIEW OF FOOD OF BALAENOPTERID WHALES AKITO KAWAMURA Faculty of Fisheries, Hokkaido University, Hakodate, Hokkaido ABSTRACT In order to elucidate what species among so many kind of marine organ isms are likely to be consumed largely by the balaenopterid whales, the ex isting evidence on the food habits of baleen whales is reviewed. To meet with this primary purpose the report was mainly focussed on to describe qualitative aspects of food species having been known to date from the notable whaling grounds over the world rather than documenting quantitative subjects.' One of interesting facts noticed throughout the contribution was that there exists fairly intense diversity in the assembly of food species composition by regions such as; northern hemisphere vs. southern hemisphere, Pacific region vs. Atlantic region, inshore waters vs. offshore waters, embayed waters vs. open waters, where the former usually shows more div'ersed complexity than the latter. The fact however suggests that although the composition of food spe cies locally varies over the various whaling grounds, the food organisms as taxonomical groups are very similar one another even in locally isolated whal ing grounds when the food organisms and their assemblies are considered by the family or genus basis. In this connection many evidences given in the text may suggest that the balaenopterid whales as a whole may substantially live on quite simply compositioned forage assembly in comparison with tre mendous variety of organisms existing in the marine ecosystems. One of im portant aspects of the baleen whales food must be found in their characteris tics of forming dense swarms, schools, and/or aggregations in the shallower enough layers to be fed by the whales. -
Retrophylogenomics in Rorquals Indicate Large Ancestral Population Sizes and a Rapid Radiation Lammers Et Al
Retrophylogenomics in rorquals indicate large ancestral population sizes and a rapid radiation Lammers et al. Lammers et al. Mobile DNA (2019) 10:5 https://doi.org/10.1186/s13100-018-0143-2 Lammers et al. Mobile DNA (2019) 10:5 https://doi.org/10.1186/s13100-018-0143-2 RESEARCH Open Access Retrophylogenomics in rorquals indicate large ancestral population sizes and a rapid radiation Fritjof Lammers1,2,3, Moritz Blumer1, Cornelia Rücklé1 and Maria A. Nilsson1,2* Abstract Background: Baleen whales (Mysticeti) are the largest animals on earth and their evolutionary history has been studied in detail, but some relationships still remain contentious. In particular, reconstructing the phylogenetic position of the gray whales (Eschrichtiidae) has been complicated by evolutionary processes such as gene flow and incomplete lineage sorting (ILS). Here, whole-genome sequencing data of the extant baleen whale radiation allowed us to identify transposable element (TE) insertions in order to perform phylogenomic analyses and measure germline insertion rates of TEs. Baleen whales exhibit the slowest nucleotide substitution rate among mammals, hence we additionally examined the evolutionary insertion rates of TE insertions across the genomes. Results: In eleven whole-genome sequences representing the extant radiation of baleen whales, we identified 91,859 CHR-SINE insertions that were used to reconstruct the phylogeny with different approaches as well as perform evolutionary network analyses and a quantification of conflicting phylogenetic signals. Our results indicate that the radiation of rorquals and gray whales might not be bifurcating. The morphologically derived gray whales are placed inside the rorqual group, as the sister-species to humpback and fin whales.