Dispelling the Fears of Hydrogen in Shielding Gases for Corrosion Resistant Overlays on High Strength Steels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fundamentals of Joining Processes
Outline ME3072 – MANUFACTURING ENGINEERING II BSc Eng (Hons) in Mechanical Engineering • Introduction to Welding Semester - 4 • Fusion-Welding Processes • Solid-State Welding Processes Fundamentals of Joining • Metallurgy of Welding Processes • Weld Quality • Brazing & Soldering Prepared By : R.K.P.S Ranaweera BSc (Hons) MSc Lecturer - Department of Mechanical Engineering University of Moratuwa 2 (for educational purpose only) Joining Processes Classification of Joining Processes 3 4 1 Introduction to Welding • Attention must be given to the cleanliness of the metal surfaces prior to welding and to possible • Is a process by which two materials, usually metals oxidation or contamination during welding process. are permanently joined together by coalescence, which is induced by a combination of temperature, • Production of high quality weld requires: pressure and metallurgical conditions. Source of satisfactory heat and/or pressure Means of protecting or cleaning the metal • Is extensively used in fabrication as an alternative Caution to avoid harmful metallurgical effects method for casting or forging and as a replacement for bolted and riveted joints. Also used as a repair • Advantages of welding over other joints: medium to reunite metals. Lighter in weight and has a great strength • Types of Welding: High corrosion resistance Fusion welding Fluid tight for tanks and vessels Solid-state (forge) welding Can be altered easily (flexibility) and economically 5 6 • Weldability has been defined as the capacity of • Steps in executing welding: metal to be welded under the fabrication conditions Identification of welds, calculation of weld area by stress imposed into a specific, suitably designed structure analysis, preparation of drawings & to perform satisfactorily in the intended service. -

MTI Friction Welding Technology Brochure
Friction Welding Manufacturing Technology, Inc. All of us at MTI… would like to extend our thanks for your interest in our company. Manufacturing Technology, Inc. has been a leading manufacturer of inertia, direct drive and hybrid friction welders since 1976. We hope that the following pages will further spark your interest by detailing a number of our products, services and capabilities. We at MTI share a common goal…to help you solve your manufacturing problems in the most Table of Contents efficient way possible. Combining friction welding Introduction to Friction Welding 2 with custom designed automation, we have Advantages of MTI’s Process 3 demonstrated dramatic savings in labor and Inertia Friction Welding 4 material with no sacrifice to quality. Contact us Direct Drive & Hybrid Friction Welding 5 today to find out what we can do for you. Machine Monitors & Controllers 6 Safety Features 7 Flash Removal 7 MTI Welding Services 8 Weldable Combinations 9 Applications Aircraft/Aerospace 10 Oil Field Pieces 14 Military 16 Bimetallic & Special 20 Agricultural & Trucking 22 Automotive 28 General 38 Special Welders & Automated Machines 46 Machine Models & Capabilities 48 Friction Welding 4 What It Is Friction welding is a solid-state joining process that produces coalescence in materials, using the heat developed between surfaces through a combination of mechanically induced rubbing motion and Information applied load. The resulting joint is of forged quality. Under normal conditions, the faying surfaces do not melt. Filler metal, flux and shielding gas are not required with this process. Dissimilar Materials Even metal combinations not normally considered compatible can be joined by friction welding, such as aluminum to steel, copper to aluminum, titanium to copper and nickel alloys to steel. -

Welding Technology a Suncam Continuing Education Course
033.pdf Welding Technology A SunCam Continuing Education Course Welding Technology By Roger Cantrell www.SunCam.com Page 1 of 35 033.pdf Welding Technology A SunCam Continuing Education Course Learning Objectives This course introduces the student to the concept of developing procedures for welding and brazing. Welding and brazing variables are introduced and some example concepts for applying each variable are highlighted to pique the student’s interest and perhaps lead to further study. Upon completion of this course, the student should be able to: • Understand the concept of creating a welding/brazing procedure • Identify several commonly used welding/brazing processes • Identify the more common welding/brazing variables • Appreciate some of the considerations for applying each variable 1.0 INTRODUCTION This course highlights the basic concepts of developing a welding or brazing procedure specification (WPS/BPS). There are a number of ways to approach this subject such as by process, base material, etc. It will be convenient to organize our thoughts in the format of ASME Section IX. The various factors that might influence weld quality are identified in ASME Section IX as "Welding Variables". "Brazing Variables" are treated in a separate part of Section IX in a manner similar to welding variables. The listing of variables for welding procedures can be found in ASME Section IX, Tables QW-252 through QW-265 (a table for each process). The layout of each table is similar to Figure No. 1. www.SunCam.com Page 2 of 35 033.pdf Welding Technology A SunCam Continuing Education Course Process Variable Variation (Description) Essential Supplementary Essential Nonessential Joint Backing X Root Spacing X Base P Number X Metal G Number X Filler F Number X Metal A Number X Continued in this fashion until all relevant variables for the subject process are listed. -

Guidelines for the Welded Fabrication of Nickel-Containing Stainless Steels for Corrosion Resistant Services
NiDl Nickel Development Institute Guidelines for the welded fabrication of nickel-containing stainless steels for corrosion resistant services A Nickel Development Institute Reference Book, Series No 11 007 Table of Contents Introduction ........................................................................................................ i PART I – For the welder ...................................................................................... 1 Physical properties of austenitic steels .......................................................... 2 Factors affecting corrosion resistance of stainless steel welds ....................... 2 Full penetration welds .............................................................................. 2 Seal welding crevices .............................................................................. 2 Embedded iron ........................................................................................ 2 Avoid surface oxides from welding ........................................................... 3 Other welding related defects ................................................................... 3 Welding qualifications ................................................................................... 3 Welder training ............................................................................................. 4 Preparation for welding ................................................................................. 4 Cutting and joint preparation ................................................................... -

Gas-Shielded Arc Welding TIG Welding
Gas-Shielded Arc Welding Gas-shielded welding can be divided into the tungsten gas-shielded welding and the metal gas-shielded welding processes. The tungsten gas-shielded welding covers the processes − Tungsten plasma arc welding (PAW) − Inert-gas tungsten-arc welding (TIG), whereby TIG welding is the most widely used fusion welding process for aluminium. The plasma welding consists only of the plasma-arc welding process which works with a transferred arc. The metal shielded-gas welding is limited to the metal inert-gas welding process operating with an inert gas as shield, as well as a process combination with plasma welding (plasma metal shielded-gas welding - PMIG). The abbreviations used are: GAW Gas-shielded arc welding GMGMMA Gas-mixture shielded metal-arc welding GTAW Gas-shielded tungsten arc welding (MAGM) GMAW Gas-shielded metal arc welding MAGC CO2-shielded metal-arc welding AHW Atomic hydrogen welding NGW Narrow-gap welding CAW Constricted arc welding EGW Electro -gas welding TIG Tungsten inert-gas arc welding PMIG Plasma MIG welding MIG Metal inert-gas arc welding MAG Metal active-gas arc welding p Pulsed arc PJW Plasma jet welding sh Short arc PAW Plasma arc welding sp Spray arc PJPW Plasma jet plasma arc welding l Long arc TIG Welding Principle of TIG Welding TIG welding equipment Watercooled TIG welding torch Torch forms for TIG welding Shielding gases for welding and cutting Flow meters Flow meter for torches Effect of current and inert gas Argon consumption for TIG welding Tungsten electrodes -

Welding and Joining Guidelines
Welding and Joining Guidelines The HASTELLOY® and HAYNES® alloys are known for their good weldability, which is defined as the ability of a material to be welded and to perform satisfactorily in the imposed service environment. The service performance of the welded component should be given the utmost importance when determining a suitable weld process or procedure. If proper welding techniques and procedures are followed, high-quality welds can be produced with conventional arc welding processes. However, please be aware of the proper techniques for welding these types of alloys and the differences compared to the more common carbon and stainless steels. The following information should provide a basis for properly welding the HASTELLOY® and HAYNES® alloys. For further information, please consult the references listed throughout each section. It is also important to review any alloy- specific welding considerations prior to determining a suitable welding procedure. The most common welding processes used to weld the HASTELLOY® and HAYNES® alloys are the gas tungsten arc welding (GTAW / “TIG”), gas metal arc welding (GMAW / “MIG”), and shielded metal arc welding (SMAW / “Stick”) processes. In addition to these common arc welding processes, other welding processes such plasma arc welding (PAW), resistance spot welding (RSW), laser beam welding (LBW), and electron beam welding (EBW) are used. Submerged arc welding (SAW) is generally discouraged as this process is characterized by high heat input to the base metal, which promotes distortion, hot cracking, and precipitation of secondary phases that can be detrimental to material properties and performance. The introduction of flux elements to the weld also makes it difficult to achieve a proper chemical composition in the weld deposit. -
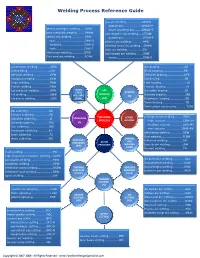
Welding Process Reference Guide
Welding Process Reference Guide gas arc welding…………………..GMAW -pulsed arc…………….……….GMAW-P atomic hydrogen welding……..AHW -short circuiting arc………..GMAW-S bare metal arc welding…………BMAW gas tungsten arc welding…….GTAW carbon arc welding……………….CAW -pulsed arc……………………….GTAW-P -gas……………………………………CAW-G plasma arc welding……………..PAW -shielded……………………………CAW-S shielded metal arc welding….SMAW -twin………………………………….CAW-T stud arc welding………………….SW electrogas welding……………….EGW submerged arc welding……….SAW Flux cord arc welding…………..FCAW -series………………………..…….SAW-S coextrusion welding……………...CEW Arc brazing……………………………..AB cold welding…………………………..CW Block brazing………………………….BB diffusion welding……………………DFW Diffusion brazing…………………….DFB explosion welding………………….EXW Dip brazing……………………………..DB forge welding…………………………FOW Flow brazing…………………………….FLB friction welding………………………FRW Furnace brazing……………………… FB hot pressure welding…………….HPW SOLID ARC Induction brazing…………………….IB STATE BRAZING WELDING Infrared brazing……………………….IRB roll welding…………………………….ROW WELDING (8) ultrasonic welding………………….USW (SSW) (AW) Resistance brazing…………………..RB Torch brazing……………………………TB Twin carbon arc brazing…………..TCAB dip soldering…………………………DS furnace soldering………………….FS WELDING OTHER electron beam welding………….EBW induction soldering……………….IS SOLDERING PROCESS WELDNG -high vacuum…………………….EBW-HV infrared soldering…………………IRS (S) -medium vacuum………………EBW-MV iron soldering……………………….INS -non-vacuum…………………….EBW-NV resistance soldering…………….RS electroslag welding……………….ESW torch soldering……………………..TS -

Laser Beams a Novel Tool for Welding: a Review
IOSR Journal of Applied Physics (IOSR-JAP) e-ISSN: 2278-4861.Volume 8, Issue 6 Ver. III (Nov. - Dec. 2016), PP 08-26 www.iosrjournals.org Laser Beams A Novel Tool for Welding: A Review A Jayanthia,B, Kvenkataramananc, Ksuresh Kumard Aresearch Scholar, SCSVMV University, Kanchipuram, India Bdepartment Of Physics, Jeppiaar Institute Of Technology, Chennai, India Cdepartment Of Physics, SCSVMV University, Kanchipuram, India Ddepartment Of Physics, P.T. Lee CNCET, Kanchipuram, India Abstract: Welding is an important joining process of industrial fabrication and manufacturing. This review article briefs the materials processing and welding by laser beam with its special characteristics nature. Laser augmented welding process offers main atvantages such as autogenous welding, welding of high thickness, dissimilar welding, hybrid laser welding, optical fibre delivery, remote laser welding, eco-friendly, variety of sources and their wide range of applications are highlighted. Significance of Nd: YAG laser welding and pulsed wave over continuous wave pattern on laser material processesare discussed. Influence of operating parameter of the laser beam for the welding process are briefed including optical fibre delivery and shielding gas during laser welding. Some insight gained in the study of optimization techniques of laser welding parameters to achieve good weld bead geometry and mechanical properties. Significance of laser welding on stainless steels and other materials such as Aluminium, Titanium, Magnesium, copper, etc…are discussed. I. INTRODUCTION Welding is the principal industrial process used for joining metals. As materials continue to be highly engineered in terms of metallic and metallurgical continuity, structural integrity and microstructure, hence, welding processes will become more important and more prominent. -

UNIT-IV Metal Joining Processes
Manufacturing Process - I UNIT –IV Metal Joining Processes Prepared By Prof. Shinde Vishal Vasant Assistant Professor Dept. of Mechanical Engg. NDMVP’S Karmaveer Baburao Thakare College of Engg. Nashik Contact No- 8928461713 E mail:- [email protected] Website:- www.vishalshindeblog.wordpress.com 06/09/2016 PROF.V.V.SHINDE NDMVP'S KBTCOE NASHIK JOINING PROCESSES • Joining includes welding, brazing, soldering, adhesive bonding of materials. • They produce permanent joint between the parts to be assembled. • They cannot be separated easily by application of forces. • They are mainly used to assemble many parts to make a system. • Welding is a metal joining process in which two or more parts are joined or coalesced at their contacting surfaces by suitable application of heat or/and pressure. • Some times, welding is done just by applying heat alone, with no pressure applied • In some cases, both heat and pressure are applied; and in other cases only pressure is applied, without any external heat. • In some welding processes a filler material is added to facilitate coalescence(Joining)06/09/2016 PROF.V.V.SHINDE NDMVP'S KBTCOE NASHIK Joining Processes: Welding, Brazing, Soldering 1. Brazing and Soldering: Melting of filler rod only • Brazing: higher temperature, ~brass filler, strong • Soldering: lower temp, ~tin-lead filler, weak 2. Welding: Melting of filler rod and base metals 06/09/2016 PROF.V.V.SHINDE NDMVP'S KBTCOE NASHIK Advantages of welding: • Welding provides a permanent joint. • Welded joint can be stronger than the parent materials if a proper filler metal is used that has strength properties better than that of parent base material and if defect less welding is done. -

Download Shielding Gas Selector Brochure(PDF 2.0
3 Product Information Shielding Gas The right gas working for you 02 Contents Contents. 3 Choosing the right gas 6 Welding processes 8 Product range 10 Gases for carbon and low-alloy steels 14 Gases for stainless steels 18 Gases for aluminium, copper and titanium alloys 20 Plasma welding and cutting 21 MIG brazing 22 Purging or root backing 24 Frequently asked questions 26 Health and safety 28 Supply options 30 Services ACCURA®, CORGON®, CRONIGON®, LASGON®, LINDATA®, LIPROTECT®, LISY®tec MISON® SECCURA® and VARIGON® are registered trademarks of the Linde Group. Choosing the right gas 03 Can a shielding gas affect the weld quality? Choosing the right gas. Ar Ar/He The addition of helium to the shielding gas results in a hotter welding arc than that produced from pure argon. Carbon dioxide Argoshield Heavy Argoshield Universal 17.1g 8.6g 8.6g For many people, the sole role of the shielding gas Weld metal properties is to protect the finished weld from the effects of Although the weld metal properties are primarily controlled by the oxygen and nitrogen in the atmosphere. What isn’t composition of the consumable, the shielding gas can influence the widely understood is that selecting the right shielding weld’s strength, ductility, toughness and corrosion resistance. gas for the job can bring many more benefits. Adding oxygen and/or carbon dioxide to a shielding gas for MIG welding carbon steel increases its oxidation potential. In general, for The choice of the shielding gas can affect: a given welding wire, the higher the oxidation potential of a shielding • the weld metal properties, such as strength, corrosion resistance gas, the lower the strength and toughness of the weld. -

Boilermaking Manual. INSTITUTION British Columbia Dept
DOCUMENT RESUME ED 246 301 CE 039 364 TITLE Boilermaking Manual. INSTITUTION British Columbia Dept. of Education, Victoria. REPORT NO ISBN-0-7718-8254-8. PUB DATE [82] NOTE 381p.; Developed in cooperation with the 1pprenticeship Training Programs Branch, Ministry of Labour. Photographs may not reproduce well. AVAILABLE FROMPublication Services Branch, Ministry of Education, 878 Viewfield Road, Victoria, BC V9A 4V1 ($10.00). PUB TYPE Guides Classroom Use - Materials (For Learner) (OW EARS PRICE MFOI Plus Postage. PC Not Available from EARS. DESCRIPTORS Apprenticeships; Blue Collar Occupations; Blueprints; *Construction (Process); Construction Materials; Drafting; Foreign Countries; Hand Tools; Industrial Personnel; *Industrial Training; Inplant Programs; Machine Tools; Mathematical Applications; *Mechanical Skills; Metal Industry; Metals; Metal Working; *On the Job Training; Postsecondary Education; Power Technology; Quality Control; Safety; *Sheet Metal Work; Skilled Occupations; Skilled Workers; Trade and Industrial Education; Trainees; Welding IDENTIFIERS *Boilermakers; *Boilers; British Columbia ABSTRACT This manual is intended (I) to provide an information resource to supplement the formal training program for boilermaker apprentices; (2) to assist the journeyworker to build on present knowledge to increase expertise and qualify for formal accreditation in the boilermaking trade; and (3) to serve as an on-the-job reference with sound, up-to-date guidelines for all aspects of the trade. The manual is organized into 13 chapters that cover the following topics: safety; boilermaker tools; mathematics; material, blueprint reading and sketching; layout; boilershop fabrication; rigging and erection; welding; quality control and inspection; boilers; dust collection systems; tanks and stacks; and hydro-electric power development. Each chapter contains an introduction and information about the topic, illustrated with charts, line drawings, and photographs. -

MSL Engineering Limited Platinum Blue House 1St Floor, 18 the Avenue Egham, Surrey, TW20 9AB
SMR Final Report 121404 Purpose of Issue Rev Date of Issue Author Agreed Approved Issued for information 0 Aug 2004 SM Issued for internal comment 1 November 2004 AFD DJM JB Issued as Final Report 2 December 2004 AFD DJM JB This Final report has been reviewed and approved by the Mineral Management Service. Approval does not signify that the contents necessarily reflect the views and policies of the Service, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This study was funded by the Mineral Management Service, U.S. Department of the Interior, Washington, D.C., under Contract Number 1435-01-04-CT-35320 ASSESSMENT OF REPAIR TECHNIQUES FOR AGEING OR DAMAGED STRUCTURES Project #502 DOC REF C357R001 Rev 1 NOV 2004 MSL Engineering Limited Platinum Blue House 1st Floor, 18 The Avenue Egham, Surrey, TW20 9AB Tel: +44 (0)1784 439194 Fax: +44 (0)1784 439198 E-mail: [email protected] C357R001Rev 2, December 2004 MMS Project #502 NUMBER DETAILS OF REVISION 0 Issued for information, August 2004 1 Issued for comment, November 2004. Extensive revisions throughout, including restructuring of report. 2 Issued as Final Report, December 2004. Conversion table added, Figure showing clamp details to avoid added, and general editorial revisions. C357R001Rev 2, December 2004 MMS Project #502 Assessment of Repair Techniques for Ageing or Damaged Structures By Dr. Adrian F Dier MSL Services Corporation Final Project Report: ASSESSMENT OF REPAIR TECHNIQUES FOR AGEING OR DAMAGED STRUCTURES MMS Project Number 502 November 2004 C357R001Rev 2, December 2004 i This Final report has been reviewed a nd approved by the Mineral Management Service.