Microcytic Anemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Training in the Biomedical, Behavioral and Clinical Sciences
RESEARCH TRAINING IN THE BIOMEDICAL, BEHAVIORAL, AND CLINICAL RESEARCH SCIENCES Committee to Study the National Needs for Biomedical, Behavioral, and Clinical Research Personnel Board on Higher Education and Workforce Policy and Global Affairs THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This project was supported by Contract/Grant No. DHHS-5294, Task Order #187 between the National Acad- emy of Science and the National Institutes of Health, Department of Health and Human Services. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the Committee to Study the National Needs for Biomedical, Behavioral, and Clinical Research Personnel and do not necessarily reflect the views of the organizations or agencies that provided support for the project. International Standard Book Number-13: 978-0-309-15965-4 (Book) International Standard Book Number-10: 0-309-15965-2 (Book) International Standard Book Number-13: 978-0-309-15966-1 (PDF) International Standard Book Number-10: 0-309-15966-0 (PDF) Library of Congress Control Number: 2011921184 Additional copies of this report are available from The National Academies Press, 500 Fifth Street, N.W., Lockbox 285, Washington, DC 20055; (800) 624-6242 or (202) 334-3313 (in the Washington metropolitan area); Internet, http://www.nap.edu. -

BPS White Paper
BOARD OF PHARMACY SPECIALTIES WHITE PAPER Five‐Year Vision for Pharmacy Specialties Approved by the BPS Board of Directors on January 12, 2013 © Copyright 2013, Board of Pharmacy Specialties Introduction The Joint Commission of Pharmacy Practitioners’ (JCPP) Future Vision of Pharmacy Practice 2015 states, “Pharmacists will be the healthcare professionals responsible for providing patient care that ensures optimal medication therapy outcomes.” i As the scope of pharmacy practice evolves to meet the complex medication‐related needs of patients, board certification is critical to assure stakeholders of the level of knowledge and skills of pharmacists who provide direct patient care. The Board of Pharmacy Specialties (BPS) acknowledges the support and collaboration of many national pharmacy organizations in this pursuit and assumes the responsibility for recognition of pharmacy specialization through board certification.ii,iii, iv In assuming this responsibility, BPS also acknowledges the pharmacist’s role in direct patient care as it continues to evolve against a backdrop of many unknowns such as changing health care delivery systems and payment models in which the role of government and the private sector are in a state of flux. Whatever changes come to fruition in the delivery of health care, BPS must move forward in a flexible manner that meets its mission to improve patient care by promoting the recognition and value of specialized training, knowledge, and skills in pharmacy and specialty board certification of pharmacists. v This white paper adds focus and clarity to how BPS will approach the board certification of pharmacist specialists over the next five years. This timeline is critical as the goal will be to create a scalable foundation on which to build future board certification activities for pharmacists. -
![PHYSICIAN-SCIENTIST [MD/PHD] Who Better to Study Disease Than Those Who Know It Intimately? WHAT IS a PHYSICIAN SCIENTIST?](https://docslib.b-cdn.net/cover/7441/physician-scientist-md-phd-who-better-to-study-disease-than-those-who-know-it-intimately-what-is-a-physician-scientist-217441.webp)
PHYSICIAN-SCIENTIST [MD/PHD] Who Better to Study Disease Than Those Who Know It Intimately? WHAT IS a PHYSICIAN SCIENTIST?
PHYSICIAN-SCIENTIST [MD/PHD] Who better to study disease than those who know it intimately? WHAT IS A PHYSICIAN SCIENTIST? A physician scientist is a unique clinician who is part of a small cadre of physicians who usually work in academic health science centers and have a high impact on health care through discovery, translational and clinical research, and clinical practice. A physician scientist in Pathology and Laboratory Medicine is a laboratory physician who is trained in both scientific biomedical investigation and in pathology and/or laboratory medicine, often with clinical subspecialty training. On one hand, the physician scientist brings the rigors of scientific investigation into the patient care arena and on the other, the physician scientist’s contact with disease brings clinically relevant questions into the research arena to drive investigations into pathogenesis, prevention, diagnosis, prognosis, and treatment of disease. asip.org/CareerPath/ MEET ADEL MAHMOUD, MD, PHD P HYSICIAN . SCIENTIST. PROFESSOR . MENTOR . Princeton University Lecturer with rank of Professor in Molecular Biology and Public Policy, Woodrow Wilson School MD from the University of Cairo in 1963 PhD from the University of London, School of Hygiene and Tropical Medicine in 1971 Elected to membership of the American Society for Clinical Investigation in 1978, the Association of American Physicians in 1980 and the Institute of Medicine of the National Academy of Sciences in 1987. Received the Bailey K. Ashford Award of the A merican Society of Tropical Medicine and Hygiene in 1983, and the Squibb Award of the Infectious Diseases Society of America in 1984 . F e l l o w of the American College of Physicians and a member of the Expert Advisory Panel on Parasitic Diseases of the World Health Organization. -

Educational Goals & Objectives the Ambulatory Medicine Rotation Will
Educational Goals & Objectives The Ambulatory Medicine rotation will provide the resident with an opportunity to become skilled in the prevention, evaluation and management of acute and chronic medical conditions commonly seen in the outpatient setting. Residents will rotate through their Ambulatory Clinic, spending increasing amounts of time throughout their 3 years in the program. They will grow their own patient panel, with patients ranging from newborns through geriatrics. The focus will be on the doctor-patient relationship, continuity of care, and the effective delivery of primary care. Residents will gain exposure to a broad spectrum of medical conditions, ranging from core internal medicine issues to conditions requiring knowledge of allergy and immunology, nutrition, obstetrics and gynecology, ophthalmology, orthopedics, otolaryngology, preventative medicine, and psychiatry as they pertain to the general care of their outpatients in the community. This exposure will complement directed subspecialty-based experiences on other rotations. They will also learn about billing and coding, insurance coverage, Patient Centered Medical Home, and other concepts pertinent to systems-based practice in the outpatient setting. Faculty will facilitate learning in the 6 core competencies as follows: Patient Care and Procedural Skills I. All residents must be able to provide compassionate, culturally-sensitive care for their clinic patients. R2s should seek directed and appropriate specialty consultation when necessary to further patient care. R3s should be able to coordinate input from multiple consultants and manage conflicting recommendations. II. All residents will demonstrate the ability to take a complete medical history and incorporate information from the electronic medical record. R1s should be able to differentiate between stable and unstable symptoms and elicit risk factors for the development of chronic disease. -

Ambulatory and Primary Care What Is Ambulatory Care?
© Ambulatory and Primary Care 1 © Presentation Objectives o Define ambulatory care o Define primary care o Explain subsets of ambulatory care o Explain ambulatory care and accreditation o Challenges and future of ambulatory care 2 © What is Ambulatory Care? oDefine ambulatory care oDefine primary care oExplain subsets of ambulatory care oExplain ambulatory care and accreditation oChallenges and future of ambulatory care 3 1 © What is Ambulatory care? • Personal health care provided to individuals who are not occupying a bed in a health care institution or in a health facility. • Ambulatory care vs. primary care • Follow-up care following inpatient episodes • A contemporaneous shift to ambulatory care 4 © Where is Ambulatory Care Service Provided? In a variety of settings, including: Freestanding provider offices Hospital-based clinics School-based clinics Public health clinics Community health centers 5 © Ambulatory Care Visits Number of Ambulatory Care Visits 7.5 7.5 8 7 6 393.9 5 3.1 4 2.1 3 1.5 2 1 0 15‐24 25‐44 75+ Male Female 6 Source: Health United States 2000 (1998 data) 2 © Physician Office Visit (National Center for Health Statistics. 2006) 7 © Physician Office Visit (National Center for Health Statistics. 2006) 8 © Physician Office Visit Data‐1: (National Center for Health Statistics. 2006) Trend of Office Visit by Type of illness and Season 9 3 © Ambulatory Care Visits: Physician‐visits by Race Number of Ambulatory Visits by Race 90 80 70 60 50 40 30 20 10 0 ER Hospital Outpatient Physician's Office Black White Other 10 © Annual rate of visits to office‐based physicians by patient race and ethnicity 11 (National Center for Health Statistics. -

SPECIAL ARTICLE Child Neurology in the 20Th Century
0031-3998/03/5302-0345 PEDIATRIC RESEARCH Vol. 53, No. 2, 2003 Copyright © 2003 International Pediatric Research Foundation, Inc. Printed in U.S.A. SPECIAL ARTICLE A History of Pediatric Specialties In the fourth article of this series, Drs. Ashwal and Rust describe the evolution of child neurology from its parent disciplines of Pediatrics and Neurology. They also document the advances that have been made in each of the many neurologic diseases of childhood. The advancement of technology and the genomic revolution have opened new pathways of research. The remarkable progress of Child Neurology in the 20th century has provided the foundation for further understanding, prevention, and treatment of the many neurologic disorders of childhood. Alvin Zipursky Editor-in-Chief Child Neurology in the 20th Century STEPHEN ASHWAL AND ROBERT RUST Department of Pediatrics [S.A.], Loma Linda University School of Medicine, Loma Linda, California 92350, U.S.A.; Departments of Neurology and Pediatrics [R.R.], The University of Virginia School of Medicine, Charlottesville, Virginia 22908-0394, U.S.A. ABSTRACT Although considered a relatively new subspecialty, child neurological conditions, and neuromuscular diseases. They have neurology traces its origins to the Hippocratic descriptions of also led to a better understanding of the neurobiologic basis of seizures and other neurologic conditions in children. Its true common problems such as global developmental delay, cerebral beginnings can be traced to the 1600s and 1700s with classical palsy, and autism. As remarkable as the advances have been in descriptions of chorea, hydrocephalus, spina bifida, and polio. It the past century, the accelerating pace of our understanding of was, however, the remarkable clinical and scientific advances in the fundamental mechanisms responsible for brain development neurology and pediatrics at the end of the 19th century that will lead to even greater achievements in the clinical care of helped create its scientific foundation. -

Cortical Superficial Siderosis and First-Ever Cerebral Hemorrhage in Cerebral Amyloid Angiopathy
Cortical superficial siderosis and first-ever cerebral hemorrhage in cerebral amyloid angiopathy Andreas Charidimou, ABSTRACT MD, PhD, MSc Objective: To investigate whether cortical superficial siderosis (cSS) is associated with increased Gregoire Boulouis, MD, risk of future first-ever symptomatic lobar intracerebral hemorrhage (ICH) in patients with cere- MSc bral amyloid angiopathy (CAA) presenting with neurologic symptoms and without ICH. Li Xiong, MD Methods: Consecutive patients meeting modified Boston criteria for probable CAA in the absence Michel J. Jessel, BS of ICH from a single-center cohort were analyzed. cSS and other small vessel disease MRI Duangnapa markers were assessed according to recent consensus recommendations. Patients were followed Roongpiboonsopit, prospectively for future incident symptomatic lobar ICH. Prespecified Cox proportional hazard MD, MSc models were used to investigate cSS and first-ever lobar ICH risk adjusting for potential Alison Ayres, BA confounders. Kristin M. Schwab, BA Jonathan Rosand, MD, Results: The cohort included 236 patients with probable CAA without lobar ICH at baseline. cSS – MSc prevalence was 34%. During a median follow-up of 3.26 years (interquartile range 1.42 5.50 M. Edip Gurol, MD, MSc years), 27 of 236 patients (11.4%) experienced a first-ever symptomatic lobar ICH. cSS was p 5 Steven M. Greenberg, a predictor of time until first ICH ( 0.0007, log-rank test). The risk of symptomatic ICH at 5 – MD, PhD years of follow-up was 19% (95% confidence interval [CI] 11% 32%) for patients with cSS at – Anand Viswanathan, baseline vs 6% (95% CI 3% 12%) for patients without cSS. In multivariable Cox regression MD, PhD models, cSS presence was the only independent predictor of increased symptomatic ICH risk during follow-up (HR 4.04; 95% CI 1.73–9.44, p 5 0.001), after adjusting for age, lobar cerebral microbleeds burden, and white matter hyperintensities. -

Ph.D. Program in Biomedical Sciences Student Handbook
PH.D. PROGRAM IN BIOMEDICAL SCIENCES STUDENT HANDBOOK COLLEGE OF MEDICINE FLORIDA STATE UNIVERSITY (Last revised 10/9/2018) Subject to changes as determined by the faculty Biomedical Sciences Graduate Student Handbook Page 1 TABLE OF CONTENTS 1. Introduction 1.1 Overview 1.2 BMS Graduate Program Support Staff 2. Degree Requirements 2.1 Laboratory Rotations 2.2 Course Work and Summary of Degree Requirements 2.2.1 Core Requirements 2.2.2 Electives 2.2.3 Seminars 2.2.4 Course Registration 2.2.5 Transfer Credit 2.2.6 Minimum GPA 2.2.7. Dual Degree and Certificate Programs 2.3 Research Presentations 2.4 Journal Clubs 2.5 Admission to Candidacy 2.5.1 Part I: The Preliminary Exam 2.5.2 Part II: The Research Proposal 2.5.3 Dissertation Hours 2.6 Dissertation 2.7 Publications 2.8 English Proficiency 3. Advising 3.1 AAMC Compact 3.2 Major Professor 3.3 Supervisory Committee 3.4 Conflict Resolution 3.4.1 Conflicts Not Involving Discrimination, Harassment or Sexual Harassment 3.4.2 Discrimination and Harassment 3.5 Conduct Codes 4. Evaluations 4.1 Annual Supervisory Committee Meetings 4.2 Annual Evaluation by the GPC 4.3 Dismissal from the Program 5. Financial Information 5.1 Assistantships and Fellowships 5.2 Fellowships and External Support 5.3 Employment and Activities Outside the Program 5.3.1 Outside Employment 5.3.2 Outside Activities 5.4 Registration Fees 5.5 Florida Residency Status Biomedical Sciences Graduate Student Handbook Page 2 5.5.1 Current Florida Residents 5.5.2 Non-Florida Residents 5.6 Healthcare 6. -

2019 National Healthcare Quality and Disparities Report Measure Specifications
2019 National Healthcare Quality and Disparities Report Measure Specifications U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Agency for Healthcare Research and Quality 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov December 2020 www.ahrq.gov/research/findings/nhqrdr/index.html This document is in the public domain and may be used and reprinted without permission. Citation of the source is appreciated. Suggested citation: 2019 National Healthcare Quality and Disparities Report Measure Specifications. Rockville, MD: Agency for Healthcare Research and Quality; December 2020. Contents Introduction .................................................................................................................................... 1 Chapter 2. Access to Care ............................................................................................................... 1 2.1 Getting Appointments for Care ............................................................................................ 1 2.2 Waiting Time ......................................................................................................................... 3 2.3 Health Insurance ................................................................................................................... 9 2.4 Usual Source of Care ........................................................................................................... 16 2.5 Patient Perceptions of Need ............................................................................................... 18 Chapter -
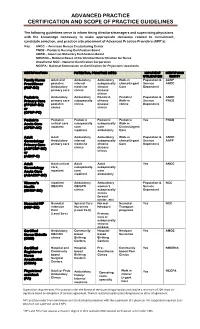
APP Scope/Practice Guide
ADVANCED PRACTICE CERTIFICATION AND SCOPE OF PRACTICE GUIDELINES The following guidelines serve to inform hiring directors/managers and supervising physicians with the knowledge necessary to make appropriate decisions related to recruitment, candidate selection, and practice site placement of Advanced Practice Providers (APP’s). Key: ANCC – American Nurses Credentialing Center PNCB – Pediatric Nursing Certification Board AMCB – American Midwifery Certification Board NBCRNA – National Board of Certification/Recertification for Nurse Anesthetist NCC – National Certification Corporation NCCPA - National Commission on Certification for Physicians' assistants CERTIFICATION POTENTIAL PRACTICE SITES INPATIENT CERTIFYING UTILIZATION ENTITY Family Nurse Adult and Ambulatory Ambulatory Walk-in Population & AANP Practitioner pediatric internal subspecialty clinics/Urgent Service ANCC (FNP-BC) Ambulatory medicine chronic Care Dependent primary care clinics disease clinics Pediatric Ambulatory Ambulatory Pediatric Pediatric Population & ANCC Primary Care primary care subspecialty chronic Walk-in Service PNCB (PPCNP-BC) pediatric clinics disease clinics Dependent or clinics clinics (CPNP-PC) Pediatric Pediatric Pediatric Pediatric Pediatric Yes PNCB Acute Care critical care subspecialty subspecialty Walk in (CPNP-AC) inpatient care care Clinics/Urgent inpatient ambulatory Care Adult Adult Ambulatory Ambulatory Walk-in Population & ANCC Gerontology Ambulatory internal subspecialty clinics/Urgent Service AAFP Primary Care primary care medicine chronic -

4 Obesity Epidemic in the United States a Cause of Morbidity and Premature Death
Morbid Obesity 61 4 Obesity Epidemic in the United States A Cause of Morbidity and Premature Death Donna M. Hunsaker, MD and John C. Hunsaker III, MD, JD CONTENTS BACKGROUND PREMATURE DEATH RELATING TO OBESITY PEDIATRIC OVERWEIGHT AND OBESITY REFERENCES SUMMARY Regarded as preeminent health issues in the Western world, overweight (OW) and obesity (OB) are overwhelmingly prevalent in the United States. Currently the United States, characterized by one writer as “Fat Land,” leads the epidemic of fat-related morbidity. Approximately 190 million Americans (approx 64% of the population) are estimated to be OW or OB. The energy balance equation defining relative homeostatic roles of energy intake and expenditure is not the sole etiological factor in a genetically defined subset of individuals with OB and OW. Beyond this subset, the energy balance is cru- cial for maintaining a healthy weight, free of significant premature natural From: Forensic Pathology Reviews, Vol. 2 Edited by: M. Tsokos © Humana Press Inc., Totowa, NJ 61 62 Hunsaker and Hunsaker disease. In the Western civilization, there is a ready supply of a high-energy, low-nutrient foods, which, when coupled with decreased physical activity, promote adiposity. OB-related complications and risks of premature death are growing concerns to the medical community as the costs of treatment for this essentially preventable condition explode continually in the wake of rising trends of OW and OB. In the adult age ranges of greater than 20 years, OW and OB have increased to 54.9% within the last decade. Life expectancy declines by 20 years if a person is OB by the age 20. -

Aspects of Knowledge, Attitudes and Practices of Medical Practitioners on Obesity and Weight Management in Three Urban Centres in Kenya
Aspects of Knowledge, Attitudes and Practices of Medical Practitioners on Obesity and Weight Management in Three Urban Centres in Kenya. Alice AChieng Ojwang Thesis presented to the Department of Human Nutrition of the University of Stellenbosch in partial fulfilment of the requirements for the degree of Masters of Nutrition Research Study Leader: Prof. D Labadarios Study Co-leader: Ms. J Visser Degree of Confidentiality: Grade C December 2005 DECLARATION OF ORIGINAL WORK I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously, in its entirety or in part, submitted it at any university for a degree. Signature December 2005 ii Abstract Objectives: To determine aspects of knowledge, attitudes and practices of Medical Practitioners on obesity and weight management in three urban centres in Kenya. Research methods and procedures: A cross sectional survey of a randomly selected sample of 485 Medical Practitioners (MPs) from three urban centres in Kenya namely Nairobi, Mombasa and Kisumu was carried out. Four hundred and thirty (89% response) questionnaires were fully completed and returned. Data was gathered on the demographics of the study population; knowledge of nutrition and obesity; awareness of obesity as a health problem assessment, management (diet, exercise and pharmacology) and practices regarding obesity as well as if and how the MPs would like their knowledge of obesity improved. Results: The MPs considered weight management as important and believed they had an important role to play. Despite the fact that some of the MPs had completed training on obesity (15%) and nutrition (53%) and nutrition respectively, they were still not confident enough to give nutrition advice to their patients.