Status Report and Assessment of Western
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poland 12 – 20 May 2018
Poland 12 – 20 May 2018 Holiday participants Gerald and Janet Turner Brian Austin and Mary Laurie-Pile Mike and Val Grogutt Mel and Ann Leggett Ann Greenizan Rina Picciotto Leaders Artur Wiatr www.biebrza-explorer.pl and Tim Strudwick. Report, lists and photos by Tim Strudwick. In Biebrza National Park we stayed at Dwor Dobarz http://dwordobarz.pl/ In Białowieża we stayed at Gawra Pensjon at www.gawra.Białowieża.com Cover photos: sunset over Lawaki fen; fallen deadwood in Białowieża strict reserve. Below: group photo taken at Dwor Dobarz and Biebrza lower basin from Burzyn. This holiday, as for every Honeyguide holiday, also puts something into conservation in our host country by way of a contribution to the wildlife that we enjoyed. The conservation contribution this year of £40 per person was supplemented by gift aid through the Honeyguide Wildlife Charitable Trust, leading to a donation of £490. The donation went to The Workshop of Living Architecture, a small NGO that runs environmental projects in and near Biebrza Marshes. This includes building new nesting platforms for white storks, often in response to storm damage or roof renovation, or simply to replace old nests. The total for all conservation contributions through Honeyguide since 1991 was £124,860 to August 2018. 2 DAILY DIARY Saturday 12 May – Warsaw to Biebrza Following an early flight from Luton, the group arrived at Warsaw airport and soon found local leader Artur and driver Rafael in the arrival hall. After introductions, including the customary carnations for the ladies, and the briefest of delays to resolve a suitcase mix up, we quickly boarded the waiting bus. -

The Mite-Y Bee: Factors Affecting the Mite Community of Bumble Bees (Bombus Spp., Hymenoptera: Apidae)
The Mite-y Bee: Factors Affecting the Mite Community of Bumble Bees (Bombus spp., Hymenoptera: Apidae) By Stephanie Margaret Haas Thesis submitted to the Faculty of Graduate and Postdoctoral Studies, University of Ottawa, in partial fulfillment of the requirements for M.Sc. Degree in the Ottawa-Carleton Institute of Biology Thèse soumise à la Faculté des études supérieures et postdoctorales, Université d’Ottawa en vue de l’obtention de la maîtrise en sciences de l’Institut de Biologie d’Ottawa-Carleton © Stephanie Haas, Ottawa, Canada, 2017 Abstract Parasites and other associates can play an important role in shaping the communities of their hosts; and their hosts, in turn, shape the community of host-associated organisms. This makes the study of associates vital to understanding the communities of their hosts. Mites associated with bees have a range of lifestyles on their hosts, acting as anything from parasitic disease vectors to harmless scavengers to mutualistic hive cleaners. For instance, in Apis mellifera (the European honey bee) the parasitic mite Varroa destructor has had a dramatic impact as one of the causes of colony-collapse disorder. However, little is known about mites associated with bees outside the genus Apis or about factors influencing the makeup of bee- associated mite communities. In this thesis, I explore the mite community of bees of the genus Bombus and how it is shaped by extrinsic and intrinsic aspects of the bees' environment at the individual bee, bee species, and bee community levels. Bombus were collected from 15 sites in the Ottawa area along a land-use gradient and examined for mites. -

Competition for Pollination and the Evolution of Flowering Time
Competition for pollination and the evolution of flowering time Item Type text; Dissertation-Reproduction (electronic) Authors Waser, Nickolas Merritt, 1948- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 29/09/2021 21:25:56 Link to Item http://hdl.handle.net/10150/565376 COMPETITION FOR POLLINATION AND THE EVOLUTION OF FLOWERING TIME bY Nickolas Merritt Waser A Dissertation Submitted to the Faculty of the DEPARTMENT OF ECOLOGY AND EVOLUTIONARY BIOLOGY In. Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY WITH A MAJOR IN BIOLOGY In the Graduate College THE UNIVERSITY OF ARIZONA 19.77 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE I hereby recommend that this dissertation prepared under my direction by Nicholas Merritt Waser___________________________ entitled COMPETITION FOR POLLINATION AND THE EVOLUTION OF FLOWERING TIME be accepted as fulfilling the dissertation requirement for the degree of Doctor of Philosophy________________________________ . uLrrt f 2 / fy\o-y^ i Dissertation Director Date As members of the Final Examination Committee, we certify that we have read this dissertation and agree that it may be presented for final defense. .2/ , ^ / f z z t fll&YoL ^ ? 4- ________ / f a / ___ ^ C c jJ jlr____ ^ Mn-J____ Final approval and acceptance of this dissertation is contingent on the candidate's adequate performance and defense thereof at the final oral examination. -

THE HUMBLE-BEE MACMILLAN and CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE the MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO the MACMILLAN CO
THE HUMBLE-BEE MACMILLAN AND CO., Limited LONDON BOMBAY CALCUTTA MELBOURNE THE MACMILLAN COMPANY NEW YORK BOSTON CHICAGO DALLAS SAN FRANCISCO THE MACMILLAN CO. OF CANADA, Ltd. TORONTO A PET QUEEN OF BOMBUS TERRESTRIS INCUBATING HER BROOD. (See page 139.) THE HUMBLE-BEE ITS LIFE-HISTORY AND HOW TO DOMESTICATE IT WITH DESCRIPTIONS OF ALL THE BRITISH SPECIES OF BOMBUS AND PSITHTRUS BY \ ; Ff W. U SLADEN FELLOW OF THE ENTOMOLOGICAL SOCIETY OF LONDON AUTHOR OF 'QUEEN-REARING IN ENGLAND ' ILLUSTRATED WITH PHOTOGRAPHS AND DRAWINGS BY THE AUTHOR AND FIVE COLOURED PLATES PHOTOGRAPHED DIRECT FROM NA TURE MACMILLAN AND CO., LIMITED ST. MARTIN'S STREET, LONDON 1912 COPYRIGHT Printed in ENGLAND. PREFACE The title, scheme, and some of the contents of this book are borrowed from a little treatise printed on a stencil copying apparatus in August 1892. The boyish effort brought me several naturalist friends who encouraged me to pursue further the study of these intelligent and useful insects. ..Of these friends, I feel especially indebted to the late Edward Saunders, F.R.S., author of The Hymen- optera Aculeata of the British Islands, and to the late Mrs. Brightwen, the gentle writer of Wild Nattcre Won by Kindness, and other charming studies of pet animals. The general outline of the life-history of the humble-bee is, of course, well known, but few observers have taken the trouble to investigate the details. Even Hoffer's extensive monograph, Die Htimmeln Steiermarks, published in 1882 and 1883, makes no mention of many remarkable can particulars that I have witnessed, and there be no doubt that further investigations will reveal more. -
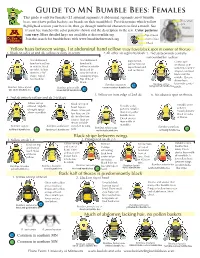
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

Scottish Bees
Scottish Bees Introduction to bees Bees are fascinating insects that can be found in a broad range of habitats from urban gardens to grasslands and wetlands. There are over 270 species of bee in the UK in 6 families - 115 of these have been recorded in Scotland, with 4 species now thought to be extinct and insufficient data available for another 2 species. Bees are very diverse, varying in size, tongue-length and flower preference. In the UK we have 1 species of honey bee, 24 species of bumblebee and the rest are solitary bees. They fulfil an essential ecological and environmental role as one of the most significant groups of pollinating insects, all of which we depend upon for the pollination of 80% of our wild and cultivated plants. Some flowers are in fact designed specifically for bee pollination, to the exclusion of generalist pollinators. Bees and their relatives Bees are classified in the complex insect order Hymenoptera (meaning membrane-winged), which also includes many kinds of parasitic wasps, gall wasps, hunting wasps, ants and sawflies. There are about 150,000 species of Hymenoptera known worldwide separated into two sub-orders. The first is the most primitive sub-order Symphyta which includes the sawflies and their relatives, lacking a wasp-waist and generally with free-living caterpillar-like larvae. The second is the sub-order Apocrita, which includes the ants, bees and wasps which are ’wasp-waisted’ and have grub-like larvae that develop within hosts, galls or nests. The sub-order Apocrita is in turn divided into two sections, the Parasitica and Aculeata. -

In Northeastern Oregon
Northwest Science Notes The purpose of Notes is to publish papers typically less than five pages long. No specific format or content is required for articles published as Notes, but all will be peer-reviewed and must be scientifically credible. Authors may contact the Editor about the suitability of manuscripts for this section. Sujaya Rao1, William P. Stephen, Department of Crop and Soil Science, Oregon State University, Corvallis, Oregon 97331 Chiho Kimoto, Department of Fisheries and Wildlife, Oregon State University, Corvallis, Oregon 97331 and Sandra J. DeBano, Department of Fisheries and Wildlife, Hermiston Agricultural Research and Extension Center, Oregon State University, Hermiston, Oregon 97838. The Status of the ‘Red-Listed’ Bombus occidentalis (Hymenoptera: Apiformes) in Northeastern Oregon Abstract The western bumble bee, Bombus occidentalis, is included on the red list of bees by The Xerces Society. It was once a common bumble bee west of the Cascades but in the late 1990s it experienced a dramatic decline along coastal regions. The cause was speculated to be due to the introduction of pathogens from captive-bred bumble bees used for pollination of greenhouse crops. In extensive surveys conducted in western and southern Oregon, 10 individuals have been recorded since 2000. In this note, we report the collection of 49 individual B. occidentalis over two years in the Zumwalt Prairie Preserve of northeastern Oregon. This finding shows that B. occidentalis persists in northeastern regions of the Pacific Northwest, either because of geographic isolation from or potential resistance to the pathogens that decimated populations in the western part of the region. Further research is needed to determine its occurrence in other regions of its historical range to assess the extent of its decline. -

The Western Bumble Bee Was Once Commonly Found N R in the Western United States and Canada
Thanks to Dr. Robbin Thorp, UC Davis. UC Thorp, Robbin Dr. to Thanks Guide developed and illustrated by Elaine Evans, The Xerces Society. Xerces The Evans, Elaine by illustrated and developed Guide Bombus appositus Bombus Bombus morrisoni Bombus Funding for bumble bee conservation provided by the CS Fund. CS the by provided conservation bee bumble for Funding Bombus melanopygus Bombus Bombus bifarius Bombus Visit www.xerces.org/bumblebees for more information. more for www.xerces.org/bumblebees Visit Bombus occidentalis Bombus , please contact [email protected] contact please , find you If FOR INVERTEBRATE CONSERVATION INVERTEBRATE FOR S X T OCIETY ERCES HE B. occidentalis B. populations. remaining will use this information to promote conservation of of conservation promote to information this use will www.xerces.org/bumblebees Society and scientists studying bumble bee decline decline bee bumble studying scientists and Society will help document their current range. The Xerces Xerces The range. current their document help will P h o in recent years. Your efforts to search for this bee bee this for search to efforts Your years. recent in t o b y D e Found in the mountains and northern areas northern and mountains the in Found r Columbia to central California have nearly disappeared disappeared nearly have California central to Columbia r Bombus huntii Bombus mixtus Bombus i c k D historic range, but populations from southern British British southern from populations but range, historic i t c h still be found in northern and eastern parts of their their of parts eastern and northern in found be still b u United States and Canada. -

Bumble Bees of the Susa Valley (Hymenoptera Apidae)
Bulletin of Insectology 63 (1): 137-152, 2010 ISSN 1721-8861 Bumble bees of the Susa Valley (Hymenoptera Apidae) Aulo MANINO, Augusto PATETTA, Giulia BOGLIETTI, Marco PORPORATO Di.Va.P.R.A. - Entomologia e Zoologia applicate all’Ambiente “Carlo Vidano”, Università di Torino, Grugliasco, Italy Abstract A survey of bumble bees (Bombus Latreille) of the Susa Valley was conducted at 124 locations between 340 and 3,130 m a.s.l. representative of the whole territory, which lies within the Cottian Central Alps, the Northern Cottian Alps, and the South-eastern Graian Alps. Altogether 1,102 specimens were collected and determined (180 queens, 227 males, and 695 workers) belonging to 30 species - two of which are represented by two subspecies - which account for 70% of those known in Italy, demonstrating the particular value of the area examined with regard to environmental quality and biodiversity. Bombus soroeensis (F.), Bombus me- somelas Gerstaecker, Bombus ruderarius (Mueller), Bombus monticola Smith, Bombus pratorum (L.), Bombus lucorum (L.), Bombus terrestris (L.), and Bombus lapidarius (L.) can be considered predominant, each one representing more than 5% of the collected specimens, 12 species are rather common (1-5% of specimens) and the remaining nine rare (less than 1%). A list of col- lected specimens with collection localities and dates is provided. To illustrate more clearly the altitudinal distribution of the dif- ferent species, the capture locations were grouped by altitude. 83.5% of the samples is also provided with data on the plant on which they were collected, comprising a total of 52 plant genera within 20 plant families. -

Rare Plant Survey of San Juan Public Lands, Colorado
Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Peggy Lyon and Julia Hanson Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 December 2005 Cover: Imperiled (G1 and G2) plants of the San Juan Public Lands, top left to bottom right: Lesquerella pruinosa, Draba graminea, Cryptantha gypsophila, Machaeranthera coloradoensis, Astragalus naturitensis, Physaria pulvinata, Ipomopsis polyantha, Townsendia glabella, Townsendia rothrockii. Executive Summary This survey was a continuation of several years of rare plant survey on San Juan Public Lands. Funding for the project was provided by San Juan National Forest and the San Juan Resource Area of the Bureau of Land Management. Previous rare plant surveys on San Juan Public Lands by CNHP were conducted in conjunction with county wide surveys of La Plata, Archuleta, San Juan and San Miguel counties, with partial funding from Great Outdoors Colorado (GOCO); and in 2004, public lands only in Dolores and Montezuma counties, funded entirely by the San Juan Public Lands. Funding for 2005 was again provided by San Juan Public Lands. The primary emphases for field work in 2005 were: 1. revisit and update information on rare plant occurrences of agency sensitive species in the Colorado Natural Heritage Program (CNHP) database that were last observed prior to 2000, in order to have the most current information available for informing the revision of the Resource Management Plan for the San Juan Public Lands (BLM and San Juan National Forest); 2. -

Meet the Rare, Threatened and Endangered Insect Pollinators Of
NDSU EXTENSION E1977 Meet the Rare, Threatened and Endangered Jeremy Hemberger Johanna James-Heinze Insect Pollinators of North Dakota David Lowenstein, Consumer Horticulture Extension Educator, Michigan State University Nathaniel Walton, Consumer Horticulture Extension Educator, Michigan State University Patrick Beauzay, Integrated Pest Management Coordinator and Research Specialist, NDSU Veronica Calles-Torrez, Post-doctoral Scientist, NDSU Gerald Fauske, Insect Collection Manager and Research Specialist, NDSU David L. Cuthrell, Esther McGinnis, Extension Horticulturist, NDSU Michigan State University Janet Knodel, Extension Entomologist, NDSU Erik Runquist, Minnesota Zoo Why are some pollinators in decline? Rusty Patched Bumble Bee (Bombus affinis) and Nectar, pollen and habitat are three major requirements of Yellow-banded Bumble Bee (B. terricola) pollinators. When habitats (for example, natural areas) are lost to Gardeners frequently see and recognize bumble bees throughout agriculture, residential homes or commercial spaces, some insect the growing season, but some species have declined rapidly in the pollinators can undergo a rapid decline. Specialized pollinators are past few decades. Two declining bumble bee species are native to more susceptible to habitat or food losses because they often are the northern U.S. from the Dakotas eastward. dependent on a few specific host plants in a specialized habitat. Both feed on specific plants, compared with other bumble bees, Environmental contamination from using herbicides that which feed on a wider host plant list. In addition to habitat loss, prevent flowers from blooming or insecticides that kill pollinators their decline may be caused by a natural disadvantage in tolerating immediately or through time degrades otherwise suitable habitats. pathogens spread from commercially reared bumble bees. -

Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan
Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan Prepared By: Logan M. Rowe, David L. Cuthrell, and Helen D. Enander Michigan Natural Features Inventory Michigan State University Extension P.O. Box 13036 Lansing, MI 48901 Prepared For: Michigan Department of Natural Resources Wildlife Division 12/17/2019 MNFI Report No. 2019-33 Suggested Citation: Rowe, L. M., D. L. Cuthrell., H. D. Enander. 2019. Assessing Bumble Bee Diversity, Distribution, and Status for the Michigan Wildlife Action Plan. Michigan Natural Features Inventory, Report Number 2019- 33, Lansing, USA. Copyright 2019 Michigan State University Board of Trustees. MSU Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover: Bombus terricola taken by D. L. Cuthrell Table of Contents Abstract ........................................................................................................................................................ iii Introduction .................................................................................................................................................. 1 Methods ........................................................................................................................................................ 2 Museum Searches ....................................................................................................................................