MT 1970 20211402 8036.Pdf (4.299Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Print This Article
International Journal of Phytomedicine 6 (2014) 384-390 http://www.arjournals.org/index.php/ijpm/index Original Research Article ISSN: 0975-0185 Heavy Metals in Selected Medicinal Plants Originated in Dana Biosphere Reserve, Jordan Mohammad Sanad Abu-Darwish1 *Corresponding author: Abs tract A study was conducted to determine the concentration of certain essential and non-essential heavy Mohammad Sanad Abu- metals (Fe, Mn, Zn, Cu, Pb and Cd) in selected medicinal plants i.e. Anemone coronaria L., Darwish Globularia arabica L., Iris petrana L., Salvia ceratophyla L. , Salvia lanigera L., and Salvia multicaulis vahl grown in their different geographic natural environment and originated in Dana Biosphere Reserve in Jordan, The concentrations of heavy metals were estimated by atomic 1 Department of Basic and Applied absorption spectrometry method. The results showed that Iron (Fe) metal recorded the highest Sciences, Shoubak University College, concentration among all studied plant samples. Its content ranged from 128.27 in A. coronaria to Al-BalqaÊApplied Universit, Al-Salt 979.64 ppm in S.lanigera. The concentration order of tested heavy metals in all studied plant 1911, Jordan. samples was Fe> Zn > Mn > Cu > Pb. The most toxic heavy metals, Pb and Cd were detected in all studied samples. Pb in all studied samples except for S. ceratophylla was lower than limits (10 ppm) reported by WHO. Concentrations of Cd in all studied plants were higher than permissible levels reported by FAO/WHO, for edible plants (0.21 ppm). Concentrations of other heavy metals particularly manganese (Mn), zinc (Zn) and Cu were also detected and were exceeded limits reported by FAO/WHO. -

These De Doctorat De L'universite Paris-Saclay
NNT : 2016SACLS250 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de doctorat (Biologie) Par Mlle Nour Abdel Samad Titre de la thèse (CARACTERISATION GENETIQUE DU GENRE IRIS EVOLUANT DANS LA MEDITERRANEE ORIENTALE) Thèse présentée et soutenue à « Beyrouth », le « 21/09/2016 » : Composition du Jury : M., Tohmé, Georges CNRS (Liban) Président Mme, Garnatje, Teresa Institut Botànic de Barcelona (Espagne) Rapporteur M., Bacchetta, Gianluigi Università degli Studi di Cagliari (Italie) Rapporteur Mme, Nadot, Sophie Université Paris-Sud (France) Examinateur Mlle, El Chamy, Laure Université Saint-Joseph (Liban) Examinateur Mme, Siljak-Yakovlev, Sonja Université Paris-Sud (France) Directeur de thèse Mme, Bou Dagher-Kharrat, Magda Université Saint-Joseph (Liban) Co-directeur de thèse UNIVERSITE SAINT-JOSEPH FACULTE DES SCIENCES THESE DE DOCTORAT DISCIPLINE : Sciences de la vie SPÉCIALITÉ : Biologie de la conservation Sujet de la thèse : Caractérisation génétique du genre Iris évoluant dans la Méditerranée Orientale. Présentée par : Nour ABDEL SAMAD Pour obtenir le grade de DOCTEUR ÈS SCIENCES Soutenue le 21/09/2016 Devant le jury composé de : Dr. Georges TOHME Président Dr. Teresa GARNATJE Rapporteur Dr. Gianluigi BACCHETTA Rapporteur Dr. Sophie NADOT Examinateur Dr. Laure EL CHAMY Examinateur Dr. Sonja SILJAK-YAKOVLEV Directeur de thèse Dr. Magda BOU DAGHER KHARRAT Directeur de thèse Titre : Caractérisation Génétique du Genre Iris évoluant dans la Méditerranée Orientale. Mots clés : Iris, Oncocyclus, région Est-Méditerranéenne, relations phylogénétiques, status taxonomique. Résumé : Le genre Iris appartient à la famille des L’approche scientifique est basée sur de nombreux Iridacées, il comprend plus de 280 espèces distribuées outils moléculaires et génétiques tels que : l’analyse de à travers l’hémisphère Nord. -

Notes on Iiving Plants from Iter Mediterraneum II Cultivated in the Botanical Garden Berlin-Dahlem
Bocconea 3 - 1992 223 Notes on Iiving plants from Iter Mediterraneum II cultivated in the Botanical Garden Berlin-Dahlem Thornas Raus Introduction When collecting monocots on the 2nd OPTIMA Expedition to Israel (19.3.-10.4.1992) I harvested a certain amount of bulbs and corms, either sterile and thus unidentified or identified but too few to be included in the expedition's generaI IO-set collection. This material was privateIy numbered and Iater incorporated into the living collection of the Botanical Garden Berlin-DahIem for further cultivation. In the meantime many of the pIants produced flowers, making determination possible. Some of the resuIts confirm relevant data as published in the generai check-list (see Danin, this volume). They are not repeated here. Others, however, represent records or taxa additional to those in the generaI check-list of Iter Mediterraneum II. They are given alphabetically in the following list. For each entry site and date of originai collection, collection number, accession number of the Botanical Garden (BG) BerIin-Dahlem, and - following the words "in flower" - the date of taking a voucher specimen (depOsited at B) are given. Some 30 gatherings, (representing the genera Allium, Arum, Bellevalia, Caralluma, Colchicum, Gagea, Muscari, and perhaps others) did not yet flower in cultivation. They are still unidentified, and consequent1y excluded from the following list. Results Allium dictyoprasum Kunth, Enum. Pl. 4: 390 (1843) Negev Highlands: Makhtesh Ramon, Karnei Ramon (34°40'OO''E;30030'20''N). Basalt outcrops, 770 m, 27.3.1989, Raus 14644 (BG Berlin-Dahlem acc. no. 138-34-89-20; in flower 21.6.1990); Kinnrot Valley (Upper Jordan Valley): 2 km NE of Kibbutz Raon (3S038'30"E - 32°44'OO"N). -

Local Adaptation in Four Iris Species Tested in a Common-Garden Experimentbij 1265 267..277
Biological Journal of the Linnean Society, 2009, 98, 267–277. With 6 figures Local adaptation in four Iris species tested in a common-garden experimentbij_1265 267..277 MICHAEL DORMAN*, YUVAL SAPIR† and SERGEI VOLIS Life Sciences Department, Ben-Gurion University of the Negev, PO Box 653, Beer Sheva 84105, Israel Received 11 February 2009; accepted for publication 25 March 2009 Local adaptation is a commonly observed result of natural selection acting in heterogeneous environment. Common-garden experiments are a method of detecting local adaptation, as well as studying phenotypic plasticity and gradients of traits. The present study aimed to analyse reaction norms of four closely-related Iris species of section Oncocyclus and to identify a role of environmentally-specific natural selection in their plastic responses. The plant vegetative and phenological, as well as performance traits were measured in a full factorial common-garden experiment with three levels of water amount and three soil types. We found a significant effect of species identity on all traits measured. Water amount and soil type affected many of the traits, but soil type did not affect the performance. There was no significant difference in the effect of water amount and soil type on performance as reflected by rhizome growth; in other words, there was no significant genotype ¥ environment interaction for performance. Plasticity levels and directions of response were also similar among the species. We conclude that phenotypic differences among species are of genetic origin, although no adaptive value was demonstrated for them at the time and life-stages ‘frame’ of this experiment. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society 2009, 98, 267–277. -

Botanická Zahrada IRIS.Indd
B-Ardent! Erasmus+ Project CZ PL LT D BOTANICAL GARDENS AS A PART OF EUROPEAN CULTURAL HERITAGE IRIS (KOSATEC, IRYS, VILKDALGIS, SCHWERTLILIE) Methodology 2020 Caspers Zuzana, Dymny Tomasz, Galinskaite Lina, Kurczakowski Miłosz, Kącki Zygmunt, Štukėnienė Gitana Institute of Botany CAS, Czech Republic University.of.Wrocław,.Poland Vilnius University, Lithuania Park.der.Gärten,.Germany B-Ardent! Botanical Gardens as Part of European Cultural Heritage Project number 2018-1-CZ01-KA202-048171 We.thank.the.European.Union.for.supporting.this.project. B-Ardent! Erasmus+ Project CZ PL LT D The. European. Commission. support. for. the. production. of. this. publication. does. not. con- stitute.an.endorsement.of.the.contents.which.solely.refl.ect.the.views.of.the.authors..The. European.Commission.cannot.be.held.responsible.for.any.use.which.may.be.made.of.the. information.contained.therein. TABLE OF CONTENTS I. INTRODUCTION OF THE GENUS IRIS .................................................................... 7 Botanical Description ............................................................................................... 7 Origin and Extension of the Genus Iris .................................................................... 9 Taxonomy................................................................................................................. 11 History and Traditions of Growing Irises ................................................................ 11 Morphology, Biology and Horticultural Characteristics of Irises ...................... -

N. S. Volume LVIII ANNALI DI BOTANICA 2000 at This Meeting The
n. s. Volume LVIII ANNALI DI BOTANICA 2000 A PRELIMINARY LIST OF RARE OR ENDANGERED IRIDACEAE SPECIES At this meeting the importance of focussing on rare and endangered Iridaceae species in each country became evident. A complete check list of the conservation status of rare and endangered Irid a cea e species is not yet estabilished. Here we present (Appendix) the list of the World Conservation Monitoring Centre (WCMC)* for rare and endangered Iridaceae, according to the IUCN categories. Some problems occur in listing rare and endangered species. For example, although in Israel legislation has protected all Iris species since 1964, so that we have now a complete list, this is not the case in other countries and for other genera. Furthermore, unsolved taxonomic problems confused the issue in some species and the exact distribution of others is still unknown. Here, we want to stimulate colleagues working on Irid a c e a e to send new information to the monitoring centre to improve the current red list. In Italy, we have species of bearded irises which are endangered because of animal and/or human influence. For example, in some areas of Italy, wild boar populations have been promoted to favour hunting, with resulting damage to Iris populations which have been rapidly reduced to a few individuals, as I. relicta Colas, on Monte delle Fate (Southern Lazio), because the boars dig up Iris rhizomes. In other areas, the intensive construction of houses has endangered some populations, for example, in the case of/, setina Colas. (Latina), a bearded Iris that flowers in February. -

Pollination of Oncocyclus Irises (Iris: Iridaceae) by Night-Sheltering Male Bees
Research Paper 417 Pollination of Oncocyclus irises (Iris: Iridaceae) by Night-Sheltering Male Bees Y. Sapir1,3, A. Shmida1, and G. Ne’eman2 1 Department of Evolution, Systematics and Ecology, The Hebrew University of Jerusalem, Givat Ram, Jerusalem, 91904, Israel 2 Dept. of Biology, University of Haifa-Oranim, Tivon, 36006, Israel 3 Present address: Dept. of Biology, Indiana University, Bloomington, IN, 47405, USA Received: January 24, 2005; Accepted: March 10, 2005 Abstract: Irises in the section Oncocyclus (Siems.) Baker (Iris: Iri- iting flowers proved to be their efficient pollinators (Kevan and daceae) grow throughout the Middle East and have large and Baker, 1983).Among the diverse groups of flower-visiting in- dark-coloured flowers but no nectar reward available to flower sects, bees are the best adapted and the most important group visitors. Consequently, no reward-collecting pollinators have of pollinators (Kevan and Baker, 1983; Proctor et al., 1996; been observed visiting the flowers during daytime. The only vis- O’Toole and Raw, 1999), and solitary bees consitute the vast itors are solitarymale bees ( Eucera spp.: Apidae) that enter the majority of bee species (Michener, 2000). flowers at dusk and staythere overnight. Here we describe the mating system of Oncocyclus irises, and the role of night-shelter- Bees are attracted to flowers mainly for the reward offered by ing male bees in their pollination system. Pollen viability in I. the flowers; this reward is mainly nectar and pollen, but other haynei on Mt. Gilboa was veryhigh (> 90%) throughout all floral types of rewards exist (Proctor et al., 1996). Bees use flowers life stages. -
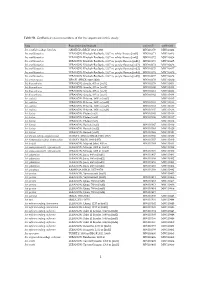
Table S1. Genbank Accession Numbers of the Iris Sequenced in This Study
Table S1. GenBank accession numbers of the Iris sequenced in this study. Taxa Population [individual] trnL-trnF matK-trnK Iris acutiloba subsp. lineolata ARMENIA (RBGK 2012-1109) MW110370 MW110422 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, white flowers [ind1] MW110371 MW110423 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, white flowers [ind2] MW110372 MW110424 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind1] MW110373 MW110425 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind2] MW110374 MW110426 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind3] MW110375 MW110427 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind4] MW110376 MW110428 Iris antilibanotica LEBANON: Kheibeh-Baalbeck, 1337 m, purple flowers [ind5] MW110377 MW110429 Iris atropurpurea ISRAEL (RBGK 1998-2808) MW110378 MW110430 Iris bismarkiana LEBANON: Sarada, 435 m [ind1] MW110379 MW110431 Iris bismarkiana LEBANON: Sarada, 435 m [ind2] MW110380 MW110432 Iris bismarkiana LEBANON: Sarada, 435 m [ind3] MW110381 MW110433 Iris bismarkiana LEBANON: Sarada, 435 m [ind4] MW110382 MW110434 Iris cedretii LEBANON: Bcharre, 1900 m [ind1] MW110435 Iris cedretii LEBANON: Bcharre, 1900 m [ind2] MW110383 MW110436 Iris cedretii LEBANON: Bcharre, 1900 m [ind3] MW110384 MW110437 Iris cedretii LEBANON: Bcharre, 1900 m [ind4] MW110385 MW110438 Iris histrio LEBANON: Ehden [ind1] MW110365 MW110416 Iris histrio LEBANON: Ehden [ind2] MW110366 MW110417 Iris histrio LEBANON: Ehden [ind3] MW110418 Iris histrio LEBANON: Barouk [ind1] MW110367 MW110419 Iris histrio LEBANON: Barouk [ind2] MW110368 MW110420 Iris histrio LEBANON: Barouk [ind3] MW110369 MW110421 Iris iberica subsp. elegantissima TURKEY: 2200 m (RBGK 1999-4347) MW110386 MW110439 Iris kirkwoodiae subsp. kirkwoodiae TURKEY (RBGK 1994-2407) MW110387 MW110440 Iris lortetii LEBANON: Mays el Jabal, 640 m MW110388 MW110441 Iris mesopotamica (I. -

Wildlife Tour Jordan Trip Report 2010
Jordan Black Irises A Greentours Tour Report March 26th – April 8th 2010 Led by Ian Green & Chris Gardner Tour report by Chris Gardner (plant list supplied by Ian Green) Day 1 March 26th UK/Turkey to Jordan We all arrived at Amman and transferred to our hotel in Madaba arriving quite late (except me as I’d flown in from Istanbul a few hours earlier). Day 2 March 27th Madaba and Dibbin The morning was essentially a cultural one dividing our time between the ornate (inside at least) Greek orthodox church with it mosaic of the holy land and then another museum with many more superb old mosaics depicting Roman life and featuring a number of recognisable birds and mammals from the time from lions to partridges. Wildlife was restricted to Greenfinches, Blackbirds, Chiffchaff and Laughing Dove whilst Peter drew our attention to the pretty grass Lamarkia aurea and there were various stork’s-bills, sandworts and pelitories. From Madaba we drove north around the outskirts of Amman and through rather foggy conditions but after a time the air cleared and we could finally see the wide valley views with many olive fields and flat-roofed houses. We saw our first drifts of deep red Ranunculus asiaticus that lined the roadsides and filled the olive groves. Many more flowers were mixed in and we spent some time here combining lunch with botanising and finding many good plants including the lilac-blue stork’s-bill Erodium gruinum, rounded ‘bushes’ of rest-harrow Ononis natrix, bluish- pink Alkanna strigosa, the robust blue-flowered and bristly Anchusa strigosa and then a rather rare orchid Ophrys bornmuelleri. -

Morphological Variation of the Oncocyclus Irises (Iris: Iridaceae) in the Southern Levant
Botanical Journal of the Linnean Society, 2002, 139, 369–382. With 3 figures Morphological variation of the Oncocyclus irises (Iris: Iridaceae) in the southern Levant YUVAL SAPIR1*, AVI SHMIDA1, ORI FRAGMAN1† and H. PETER COMES2 1Rotem – Israel Plant Information Center, Department of Evolution, Systematics and Ecology, The Hebrew University, Givat Ram, Jerusalem 91904, Israel 2Institut für Spezielle Botanik, Johannes Gutenberg-Universität, Mainz, Bentzelweg 9 A, D-55099 Mainz, Germany Received November 2001; accepted for publication April 2002 Morphological traits of Iris section Oncocyclus (Siems.) Baker in the southern Levant (Israel, Jordan, The Palestinian Authority and Sinai/Egypt) were analysed in order to clarify taxonomic relationships among taxa and the validity of diagnostic characters. Floral and vegetative characters were measured in 42 populations belonging to nine species during the peak of the flowering season in 1998–2000. Pearson’s Coefficient of Racial Likelihood (CRL) was used to calculate morphological distances between populations. Twelve of the measured populations, distributed along the north-south aridity gradient in Israel, were further explored for morphological changes along the gradient. Cluster analysis revealed two major clusters: the first includes most of the dark-coloured Iris popu- lations, with populations of I. petrana Dinsmore and I. mariae W. Barbey forming a subcluster; the second consists of all the light-coloured populations but also some dark-coloured populations. Pearson’s CRL and geographical dis- tance were significantly correlated among the dark-coloured populations. Along the geographical gradient, flower, stem and leaf size traits decrease towards the south, probably as an adaptation to aridity. This suggests that natural selection promoted the differences between populations. -

A List of Flowering Wild Plants in Tafila Province, Jordan
Vol. 6(1), pp. 28-40, January 2014 DOI: 10.5897/IJBC2011.116 International Journal of Biodiversity and ISSN 2141-243X © 2013 Academic Journals http://www.academicjournals.org/IJBC Conservation Full Length Research Paper A list of flowering wild plants in Tafila Province, Jordan Sawsan Atallah Oran Department of Biological Sciences, Faculty of Sciences, University of Jordan. Amman, Jordan. Accepted 27 November, 2013 Wild flowering plants in Tafila Province (South of Jordan) in terms of floristic features, with regards to its wild vascular plants were studied. A list of wild flowering plants was prepared. Field trips were made to the study area. A total number of 383 species belong to 198 genera and 48 families were recorded. Wild trees like Cupressus sempervirens, Ceratonia siliqua, Quercus sp. (Oak) and Juniperus phoenica were reported. Some recorded species such as Anthemis maris- mortui, or the medicinal rare species such as Iris petrana and Iris nigricans, Salvadora persica, Osyris alba, Datura stramonium, Globularia Arabica and Amygdalus communis are considered as endemic to the area. A number of historical economic trees have been recorded in the study area like Pistacia atlantica; some edible species are reported like Malva sp., Allium sp., Gundelia tournefortii, etc. Some exotic plant species were reported like Iris species and Lupinus varius. Some endemic species were reported, e.g Iris petrana, rare plant species were also recorded, e.g, Globularia arabica, I. nigricans, Iris edomensis and Limonium sp. Plant examples are listed and some selected photos for some plant species from the study area are included. Key words: Plant diversity, Jordan, Tafila, conservation. -

Black on Black
Published on Khammash Architects (https://www.khammash.com) Black on Black By Ammar Khammash, The Jordan Times Weekender - April 2003 Black is beautiful. Nature has been very stringent in using black on flowers. It is strange to see black in bloom, but when it occurs, the result is of utmost elegance. The Black Iris, Jordan?s national flower, has adapted to a landscape of ample sun. It has evolved in a harsh environment, wind-blown, to acquire a unique color matching the black eyes of remote shepherds. ?Iris Nigricans?, the scientific name of Jordan?s Black Iris, suggests its color. But Jordan has nine other different kinds of iris, so far identified in the wild. Some are extremely rare, and one kind - Iris Vartanii - is now extinct. Vartanii Iris had flowers in light blue; it was recorded in the mountains of Salt as a species new to Jordan. Dr. Dawud Al Eisawi mentions in his book, ?Wild Flowers of Jordan?, how the habitat of this flower was destroyed leading to its disappearance. If you spot some iris on the mountainsides overlooking the Jordan Valley, in regions north of Amman, such flowers would be either Jil?ad Iris -Iris Atrofusca- in the mountains between Salt and Jerash, or Purple Iris -Iris Atropurpuraea- if found around Irbid, Ajloun, or near Um Qais. To the south of Amman, you would find the real Black Iris, our national pride, in landscapes between Madaba and Karak. Its entire flower is dipped in black, as a round sphere as big as 12 to 15cm in diameter; the flower has a glossy lustrous finish, shimmering in the blowing wind.