Guidelines for Immunization of Source Plasma (Human) Donors with Blood Substances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Product Replacement: Obstetric Hemorrhage
CMQCC OBSTETRIC HEMORRHAGE TOOLKIT Version 2.0 3/24/15 BLOOD PRODUCT REPLACEMENT: OBSTETRIC HEMORRHAGE Richard Lee, MD, Los Angeles County and University of Southern California Medical Center Laurence Shields, MD, Marian Regional Medical Center/Dignity Health Holli Mason, MD, Cedars-Sinai Medical Center Mark Rollins, MD, PhD, University of California, San Francisco Jed Gorlin, MD, Innovative Blood Resources/Memorial Blood Center, St. Paul, Minnesota Maurice Druzin, MD, Lucile Packard Children’s Hospital Stanford University Jennifer McNulty, MD, Long Beach Memorial Medical Center EXECUTIVE SUMMARY • Outcomes are improved with early and aggressive intervention. • Both emergency blood release and massive transfusion protocols should be in place. • In the setting of significant obstetric hemorrhage, resuscitation transfusion should be based on vital signs and blood loss and should not be delayed by waiting for laboratory results. • Calcium replacement will often be necessary with massive transfusion due to the citrate used for anticoagulation in blood products. • During massive transfusion resuscitation, the patient’s arterial blood gas, electrolytes, and core temperature should be monitored to guide clinical management and all transfused fluids should be warmed; direct warming of the patient should be initiated as needed to maintain euthermia and to avoid added coagulopathy. BACKGROUND AND LITERATURE REVIEW After the first several units of packed red blood cells (PRBCs) and in the face of continuing or worsening hemorrhage, aggressive transfusion therapy becomes critical. This report covers the experience with massive transfusion protocols. Lessons from military trauma units as well as civilian experience with motor vehicle accidents and massive obstetric hemorrhage have identified new principles such as earlier use of plasma (FFP/thawed plasma/plasma frozen within 24 hours/liquid plasma) and resuscitation transfusion while laboratory results are pending. -

Red Blood Cells, Leukocyte Reduced Covenant and AHS Sites
This document applies to all Red Blood Cells, Leukocyte Reduced Covenant and AHS sites. Class: Human blood component, derived from OTHER NAMES: Packed red blood cells, Packed whole blood cells INTRAVENOUS OTHER ROUTES DIRECT IV Intermittent Continuous Infusion Infusion SC IM OTHER Acceptable No Yes No No No No Routes* * Professionals performing these restricted activities have received authorization from their regulatory college and have the knowledge and skill to perform the skill competently. DESCRIPTION OF PRODUCT: Red Blood Cell (RBC) concentrates are prepared from approximately 480mL whole blood usually collected in 70mL of anticoagulant. The unit is plasma reduced by centrifugation, platelet reduced by either centrifugation or filtration and leukocyte reduced by filtration. RBCs are typically resuspended in approximately 110mL of nutrient. Collected from volunteer donors by the Canadian Blood Services (CBS). Donor is screened and blood is tested for: i. Hepatitis B Surface Antigen (HBsAg) ii. Syphilis iii. Antibodies to Hepatitis B core antigen (HBcore), Hepatitis C Virus (HCV), Human T-cell Lymphotropic Virus (HTLV-1 and 2), Human Immune Deficiency Virus (HIV-1 and 2) iv. Nucleic Acid Testing (NAT) for presence of HCV RNA, HIV-1 RNA and West Nile Virus (WNV) RNA . CBS also tests blood for ABO/Rh and clinically significant antibodies. Some donors are tested for CMV status. RBCs do NOT contain any coagulation factors or platelets. AVAILABILITY: . Contact your local transfusion service/laboratory for ABO and Rh types stocked at your site. ABO compatible (not always identical) may be required if the transfusion service cannot provide sufficient quantities of the patient’s blood group (See ABO compatibility chart at: http://www.albertahealthservices.ca/assets/wf/lab/wf-lab-clin-tm-abo-compatability.pdf). -

Red Blood Cell Transfusions –
Peer Reviewed VETcpd - Internal Medicine Jenny Walton BVM&S MRCVS Jenny qualified from R(D)SVS in Red blood cell transfusions – 1998 - she worked in mixed practice when, what and how to do it! for 4 years before moving into the Red cell transfusions are now a relatively common intervention in veterinary practice field of small in the UK and help in the treatment of many patients. This is largely due to the animal emergency availability of blood products, such as Packed Red Blood Cells (PRBC) , from blood and critical care banks supplying directly to practices. After donation, red cells are separated from with Vets Now for 12 years. Through plasma into a concentrated packed cell form, a nutrient extender is then added to Vets Now, she ran the practical trial them. This allows the red cells to be stored for up to 42 days before being transfused researching canine blood banking in 2005-2006 – launching Pet Blood Bank into patients. DEA 1 blood typing prior to transfusion is essential and cross matching UK (PBB) alongside Wendy Barnett should be performed for second transfusions. Blood products are administered in 2007. She acts as the Veterinary through a filtered giving set and patients monitored closely for transfusion reactions. Supervisor for PBB, her role includes Transfusion reactions are thankfully rare but potentially life threatening. advising practitioners daily on the appropriate use of PBB blood products, Key words: Canine, Packed Red Blood Cells (PRBC), transfusion, blood typing, overseeing the practical and VMD cross matching, transfusion reactions legislative veterinary aspects of blood collection at PBB and leading research on future development opportunities. -

Canine and Feline Transfusion Medicine Lecture Notes (VALVT).Pdf
Transfusion medicine is a staple in veterinary emergency medicine that has allowed us to provide a potentially life saving treatment option to our clients and treatment to our patients. Transfusions are a process used to administer blood or blood products to a patient. By providing blood or blood products to a patient we can: treat hypoxemia and hypoxia by increasing oxygen carrying capacity, increase tissue perfusion by increasing circulating volume, improve hemostasis by delivering clotting factors, improve immune system response by delivering antiinflammatory mediators and increase protein levels by delivering protein sources. Component therapy has grown favor over the years in only administering the blood product that the patient is deficient in. This helps to treat the primary problem as well as decrease the risk of transfusion reactions or complications. Whole blood can be broken down into a variety of components: packed red blood cells, plasma, platelet rich plasma and cryoprecipitate to name a few. For the purposes of this lecture we will be focusing our discussion on the uses of whole blood, packed red blood cells and plasma. We will briefly discuss cryoprecipitate and albumin. Whether blood or blood constituents are lost it is important to replace them to regain stability in our emergent patients. Loss of packed red blood cells can be caused by immune mediated diseases like immune mediated hemolytic anemia (IMHA), a decrease in production of red blood cells as seen in chronic kidney disease (CKD), chronic inflammatory diseases like pancreatitis, bone marrow dysfunction as in leukemia or neoplasia. Other causes of red blood cell loss can be from outside sources like infectious agents such as Mycoplasma or toxins such as zinc, garlic or onion ingestion. -

Clinical Transfusion Practice
Clinical Transfusion Practice Guidelines for Medical Interns Foreword Blood transfusion is an important part of day‐to‐day clinical practice. Blood and blood products provide unique and life‐saving therapeutic benefits to patients. However, due to resource constraints, it is not always possible for the blood product to reach the patient at the right time. The major concern from the point of view of both user (recipient) and prescriber (clinician) is for safe, effective and quality blood to be available when required. Standard practices should be in place to include appropriate testing, careful selection of donors, screening of donations, compatibility testing, storage of donations for clinical use, issue of blood units for either routine or emergency use, appropriate use of blood supplied or the return of units not needed after issue, and reports of transfusion reactions – all are major aspects where standard practices need to be implemented. In order to implement guidelines for standard transfusion practices, a coordinated team effort by clinicians, blood transfusion experts, other laboratory personnel and health care providers involved in the transfusion chain, is needed. Orientation of standard practices is vital in addressing these issues to improve the quality of blood transfusion services. Bedside clinicians and medical interns are in the forefront of patient management. They are responsible for completing blood request forms, administering blood, monitoring transfusions and being vigilant for the signs and symptoms of adverse reactions. -

Red Blood Cell Transfusion a Pocket Guide for the Clinician
Red Blood Cell Transfusion A Pocket Guide for the Clinician Robert Weinstein, MD University of Massachusetts Medical School November 2016 Adapted from Red Blood Cell Transfusion: A clinical practice guideline from the AABB, Clinical Practice Guidelines from the AABB: Red blood cell transfusion thresholds and storage, and additional sources Red Blood Cells as a Therapeutic Product Irradiation Prevention of TA-GVHD in certain Radiation dose: 2500 circumstances cGy to center of product Appropriate uses of red blood cell (RBC) transfusion Donor categories Gamma or • Treatment of symptomatic anemia Product donated by family member X-irradiation • Prophylaxis in life-threatening anemia Product from HLA-selected donor Shelf life of irradiated Products from directed donors • Restoration of oxygen-carrying capacity in case of hemorrhage product: up to 28 whose relationship to recipient’s • RBCs are also indicated for exchange transfusion days unless original family has not been established w Sickle cell disease expiration date is w Severe parasitic infection (malaria, babesiosis) Pediatric practice sooner w Severe methemoglobinemia Intrauterine transfusion (IUT) NB: Supernatant K+ w Severe hyperbilirubinemia of newborn Exchange or simple transfusion in may be higher than neonates if prior IUT usual RBC transfusion is not routinely indicated for pharmacologically treatable Congenital immune deficiency Allogeneic HPC states anemia such as: transplant recipient: • Iron deficiency anemia Acute leukemia: HLA-matched or Start with initiation of • Vitamin -

Transfusion Management of Obstetric Hemorrhage E
4 Transfusion Management of Obstetric Hemorrhage E. Lockhart INTRODUCTION incidence in the US and other high resource countries underscore the importance of increasing knowledge The importance of transfusion medicine in the man- of transfusion practice among obstetricians10,11. The agement of postpartum hemorrhage (PPH) cannot be goal of this chapter is to provide a concise review of overstated and is reflected in the historical record with blood products and their use in obstetric hemorrhage, the first series of successful human-to-human transfu- with emphasis on transfusion management of massive sions being performed by James Blundell in 1818, a obstetric hemorrhage. [Editor’s note: This chapter com- London obstetrician treating patients with PPH1. prises two major sections: a thorough discussion of the various Blundell wrote the following2: components of blood transfusion products and an exceptional ‘. if you have under care a patient in whom the flooding section on management of major obstetric hemorrhage. has been copious, in whom, further, the womb has been Readers have the option to continue as their specific interests emptied, and the haemorrhages been stopped; should this direct them. L.G.K.] woman, as I have myself on several occasions seen, be sinking gradually into the grave, so that even to those who have seen much of floodings the case appears to be without hope: under such circumstances, I affirm that it is highly proper to have BLOOD TRANSFUSION PRODUCTS recourse to the operation of transfusion, provided we are Red blood cells competent to perform it.’ Product description and selection for transfusion Such prescriptions remain valid today. -
337: Alternatives to Blood Transfusions Part 1 of 2
14 | the surgical technologist | DECEMBER 2011 Alternatives to Blood Transfusions Joel massop, cst Bloodless surgery is a term that was popularized in the early 1900s by the practice of an internationally famous orthopedic surgeon, Dr Adolf Lorenz, who was known as “the bloodless surgeon of Vienna.” At that time, carbolic acid was routinely used in the operating room to clean a surgeon’s hands. Due to Lorenz’ allergic reaction to carbolic acid, he began treating his patients with non-invasive tech- niques. Thus the term “bloodless surgeon of Vienna.”10 Dr Lorenz developed a huge reputation for his ability to treat clubfeet. He was able to stretch even to the point of breaking the tendons, ligaments and epiphyseal plates until LEARNING ObJECTIVes the foot was appropriately aligned. He then would apply a ▲ Identify alternatives to blood cast until the foot healed in that position. transfusions He was also involved with ▲ List the types of methods and the treatment of procedures used as alternatives for scoliosis, but was these procedures most famous for ▲ Examine the various intraoperative his treatment of surgical techniques and congenital dis- instruments that can be used location of the hip. His tech- ▲ Recall the risks associated with nique involved these types of procedures manipulating ▲ Define hypotensive anesthesia as the hip in young related to bloodless surgery children under light anesthesia and holding them Dr Adolf Lorenz in a body cast as JANUARY 2012 | the surgical technologist | 15 they matured.10 The New York Times, in December of 1902, blood should be evaluated for anemia and any indication of wrote about a case involving double dislocation of the hip this should be treated before surgery. -

Guidelines for the Transfusion of Packed Red Blood Cells, Fresh Frozen
MANUAL: POLICY #: SUBJECT: Guidelines for the transfusion of EFFECTIVE DATE: packed red blood cells, fresh frozen REVISED DATE: plasma, platelets, and cryoprecipitate in critically ill AUTHORIZED patients APPROVAL: PERSONNEL Nursing, respiratory and physician COVERED: care providers of relevant patients in intensive care unit environments PAGE: 1 OF 4 PURPOSE The purpose of this policy is to develop a clinical practice guideline for the transfusion of packed red blood cells, fresh frozen plasma, platelets, and cryoprecipitate in adult patients in (surgical) intensive care unit environments. Blood products are a scarce resource and their transfusion may result in complications, suggesting that an evidence-based approach for practice, tailored to clinical situations, is most appropriate. DEFINITION Packed red blood cells are used to treat hemorrhage and anemia as well as to improve oxygen delivery to tissues. Fresh frozen plasma is a blood component from whole blood or collected by apheresis, frozen within time limits and at a temperature such as to preserve the labile clotting factors adequately. It is dosed at 10- 15mL/kg of body weight, depending on clinical situation in an ABO- (not necessarily Rh-) compatible fashion. Platelets are a blood component from whole blood or collected by apheresis transfused in an ABO- and Rh-compatible fashion in those with thrombocytopenia (<100,000), relative thrombocytopenia depending on circumstance (<10-100,000) or thrombocytopathia. Cryoprecipitate is a blood component containing fibrinogen and other coagulation factors (e.g. Factor 8, 13 and von Willebrand’s factor). POLICY Transfusions of packed red blood cells, fresh frozen plasma, platelets and/or cryoprecipitate will be ordered by ICU care providers in consultation with the primary team and relevant consultants. -

Transfusion Medicine & Blood Products
RED CELL PRODUCTS PRODUCT CHARACTERISTICS INDICATIONS Whole blood 450 mL. Coagulation Provides oxygen-carrying capacity and blood volume not (not available at factors adequate, platelets generally available Shands) low in number Packed red cells 250-300 mL, contain Provide red cells for oxygen-carrying capacity leukocytes and platelets sufficient for allo- immunization; can be stored up to 35 or 42 days at 40C Leukocyte-depleted Like packed red cells but Reduce incidence of febrile reactions (especially if red cells contain less than 5x106 leukodepletion performed pre-storage); reduce the risk of leukocytes HLA alloimmunization and CMV transmission Deglycerolized 200 mL Used when cells with rare blood group phenotypes are frozen red cells required; prevention of anaphylaxis in patients with anti- lgA antibodies Washed red cells Saline-suspended red cells Useful for patients with paroxysmal nocturnal in 200-250 mL hemoglobinuria, severe allergic reactions to donor plasma proteins, or non-hemolytic febrile reaction Irradiated red blood Like packed red blood cells Used to reduce lymphocytes and prevent GVHD in those at cells risk (HSCT patients, aplastic anemia, directed donation) FEVER ASSOCIATED WITH TRANSFUSION OF BLOOD COMPONENTS DEFINITION: Rise of at least 10 C during or within 1-2 hours of the transfusion Acute hemolytic transfusion reaction Bacterial sepsis Febrile non-hemolytic transfusion reaction Unrelated to the transfusion APPROACH TO FEVER ASSOCIATED WITH TRANSFUSION: General considerations Stop the transfusion Keep the intravenous -

A Compendium of Transfusion Practice Guidelines Edition 4.0 January 2021
A Compendium of Transfusion Practice Guidelines Edition 4.0 January 2021 Table of Contents Introduction Red Blood Cells Platelets Low Titer Group O Whole Blood Plasma Cryoprecipitated AHF Testing Serices Therapeutic Apheresis Blood Component Modifications Hospital Transfusion Committee Patient Blood Management Appendices Introduction Transfusion of blood products is one of the most common medical procedures, used for patients with a wide range of medical conditions to improve tissue oxygenation, achieve hemostasis, and/or fight infections. Moreover, transfusion therapy enables many complex procedures, such as organ transplantation, cardiac and other surgeries, and stem cell transplantation. Yet the curriculum of academic medical and nursing programs provide limited exposure and training towards helping providers to understand the attributes of the different types of blood products, appreciate the risks of transfusion, and raise awareness of the accumulating body of evidence that is helping to refine our understanding of clinical indications for each type of transfusion. While the approach to transfusion medicine has historically been based on personal experience, local practice, expert opinion, and consensus conference recommendations, the availability of hemovigilance data that document the adverse effects of transfusion, randomized controlled trials (RCTs) demonstrating both the benefits and risks of transfusion, and growing debates regarding alternate therapies provide a good foundation to develop evidence-based resources to aid in transfusion care of today and the future. There is a growing belief that transfusion therapy, like many other types of drug therapies, can be tailored or personalized to address specific patient and disease contexts. One example is in the care of the actively hemorrhaging patient, particularly in the prehospital time frame. -
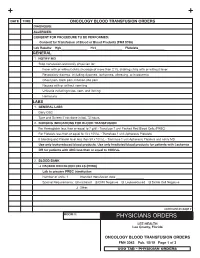
3043 Oncology Transfusion Orders
Date tiMe oncology blooD transfusion orDers Diagnosis: allergies: consent for ProceDure to be PerforMeD: consent for transfusion of blood or blood Products (fM# 5766) lab results: Hgb_________________ Hct_________________ Platelets_________________ general 1. notify MD Stop transfusion and notify physician for: Fever with or without chills (increase of more than 2˚ F), shaking chills with or without fever Respiratory distress, including dyspnea, tachypnea, wheezing, or hypoxemia Chest pain, back pain, infusion site pain Nausea with or without vomiting Urticaria including hives, rash, and itching Hematuria labs 1. general labs Daily CBC Type and Screen if not done in last 72 hours 2. nursing inDications for blooD transfusion For Hemoglobin less than or equal to 7 g/dl - Transfuse 1 unit Packed Red Blood Cells (PRBC) For Platelets less than or equal to 10 x 103/uL - Transfuse 1 unit Apheresis Platelets If bleeding and Platelet level less than 50 x 103/uL - Transfuse 1 unit Apheresis Platelets and notify MD use only leukoreduced blood products. use only irradiated blood products for patients with leukemia or for patients with anc less than or equal to 1000/ul. 2. blooD bank q PackeD reD blooD cells (Prbc) lab to prepare Prbc transfusion Number of units: 1 Intended transfusion date __________________ Special Requirements: q Irradiated q CMV Negative q Leukoreduced q Sickle Cell Negative q Other: continued on page 2 rooM #: PHYSICIANS ORDERS lee HealtH lee county, florida oncology blooD transfusion orDers fM# 3043 Pub. 10/19 Page 1 of 3 UCO