Source Identification and Health Risks Assessment of Toxic Metals in Rainwater and Groundwater in Eket and Esit Eket, Nigeria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
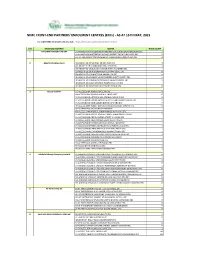
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (Ercs) - AS at 15TH MAY, 2021
NIMC FRONT-END PARTNERS' ENROLMENT CENTRES (ERCs) - AS AT 15TH MAY, 2021 For other NIMC enrolment centres, visit: https://nimc.gov.ng/nimc-enrolment-centres/ S/N FRONTEND PARTNER CENTER NODE COUNT 1 AA & MM MASTER FLAG ENT LA-AA AND MM MATSERFLAG AGBABIAKA STR ILOGBO EREMI BADAGRY ERC 1 LA-AA AND MM MATSERFLAG AGUMO MARKET OKOAFO BADAGRY ERC 0 OG-AA AND MM MATSERFLAG BAALE COMPOUND KOFEDOTI LGA ERC 0 2 Abuchi Ed.Ogbuju & Co AB-ABUCHI-ED ST MICHAEL RD ABA ABIA ERC 2 AN-ABUCHI-ED BUILDING MATERIAL OGIDI ERC 2 AN-ABUCHI-ED OGBUJU ZIK AVENUE AWKA ANAMBRA ERC 1 EB-ABUCHI-ED ENUGU BABAKALIKI EXP WAY ISIEKE ERC 0 EN-ABUCHI-ED UDUMA TOWN ANINRI LGA ERC 0 IM-ABUCHI-ED MBAKWE SQUARE ISIOKPO IDEATO NORTH ERC 1 IM-ABUCHI-ED UGBA AFOR OBOHIA RD AHIAZU MBAISE ERC 1 IM-ABUCHI-ED UGBA AMAIFEKE TOWN ORLU LGA ERC 1 IM-ABUCHI-ED UMUNEKE NGOR NGOR OKPALA ERC 0 3 Access Bank Plc DT-ACCESS BANK WARRI SAPELE RD ERC 0 EN-ACCESS BANK GARDEN AVENUE ENUGU ERC 0 FC-ACCESS BANK ADETOKUNBO ADEMOLA WUSE II ERC 0 FC-ACCESS BANK LADOKE AKINTOLA BOULEVARD GARKI II ABUJA ERC 1 FC-ACCESS BANK MOHAMMED BUHARI WAY CBD ERC 0 IM-ACCESS BANK WAAST AVENUE IKENEGBU LAYOUT OWERRI ERC 0 KD-ACCESS BANK KACHIA RD KADUNA ERC 1 KN-ACCESS BANK MURTALA MOHAMMED WAY KANO ERC 1 LA-ACCESS BANK ACCESS TOWERS PRINCE ALABA ONIRU STR ERC 1 LA-ACCESS BANK ADEOLA ODEKU STREET VI LAGOS ERC 1 LA-ACCESS BANK ADETOKUNBO ADEMOLA STR VI ERC 1 LA-ACCESS BANK IKOTUN JUNCTION IKOTUN LAGOS ERC 1 LA-ACCESS BANK ITIRE LAWANSON RD SURULERE LAGOS ERC 1 LA-ACCESS BANK LAGOS ABEOKUTA EXP WAY AGEGE ERC 1 LA-ACCESS -

Eket, Cross River State
Problems of industrial fisheries development in the Cross River State Item Type conference_item Authors Essien, J. Download date 24/09/2021 14:50:33 Link to Item http://hdl.handle.net/1834/21086 14 PRO3LEMS OF INDUSTRIAL TISHERXES DEVELOPMENT IN THE CROSS RIVER STATE by James Essico Seastate Seafoods Ltd., Eket, CrossRiver State A S S T R A C T T1i paper describes briefly the industrial fisheries as opposed to artisanal fisheries in the Cross River State. It gives a brief description on the prospect of industrial fisheries in the State and proceeds further to identify the major fish and shrimp resources within the coastal waters. It dosis briefly on the introduction of industrial fishing in 1973 when the State Government invited Japaneese Company to carry out a joint exploratory shrimp fishing venture and also the government's oferte to acquire a stern trawler for exploratory fishing. The contributions made by the Seastate Seafoods Company, the Eyib's Nutritional Food and the Arawak Fishing Companies towards the increase in the number of fishing fleet in the State are noted. the major causes of the decline in the industrial fisheries are briefly discussed. These include the management problems, inadequate tinancing, lack of civalified personnel to operate the vessels, poor uaíntsnance facility and lack of supporting infrastructure. it discusses the effects of the development of offshore oil on coastal fisheries, The recommendations include supervised credit, development of basic infrastructure and boat building, standardising cquipment for fishing vessels andprotection of our coastRi waters. LNTROI)UCT ION ?ishing industry in the Cross River State is dominated by the artisanal fisheries which involves the use of canoes both mechanised end unmechanised. -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

NIGERIA: Registration of Cameroonian Refugees September 2019
NIGERIA: Registration of Cameroonian Refugees September 2019 TARABA KOGI BENUE TAKUM 1,626 KURMI NIGERIA 570 USSA 201 3,180 6,598 SARDAUNA KWANDE BEKWARA YALA DONGA-MANTUNG MENCHUM OBUDU OBANLIKU ENUGU 2,867 OGOJA AKWAYA 17,301 EBONYI BOKI IKOM 1,178 MAJORITY OF THE ANAMBRA REFUGEES ORIGINATED OBUBRA FROM AKWAYA 44,247 ABI Refugee Settlements TOTAL REGISTERED YAKURR 1,295ETUNG MANYU REFUGEES FROM IMO CAMEROON CROSS RIVER ABIA BIOMETRICALLY BIASE VERIFIED 35,636 3,533 AKAMKPA CAMEROON Refugee Settlements ODUKPANI 48 Registration Site CALABAR 1,058MUNICIPAL UNHCR Field Office AKWA IBOM CALABAR NDIAN SOUTH BAKASSI667 UNHCR Sub Office 131 58 AKPABUYO RIVERS Affected Locations 230 Scale 1:2,500,000 010 20 40 60 80 The boundaries and names shown and the designations used on this map do not imply official Kilometers endorsement or acceptance by the United Nations. Data Source: UNHCR Creation Date: 2nd October 2019 DISCLAIMER: The boundaries and names shown, and the designations used on this map do not imply official endorsement or acceptance by the United Nations. A technical team has been conducting a thorough review of the information gathered so as to filter out any data discrepancies. BIOMETRICALLY VERIFIED REFUGEES REGISTRATION TREND PER MONTH 80.5% (35,636 individuals) of the total refugees 6272 counteded at household level has been 5023 registered/verified through biometric capture of iris, 4025 3397 fingerprints and photo. Refugee information were 2909 2683 2371 also validated through amendment of their existing 80.5% information, litigation and support of national 1627 1420 1513 1583 586 VERIFIED documentations. Provision of Refugee ID cards will 107 ensure that credible information will effectively and efficiently provide protection to refugees. -

Nigeria Vision 20: 2020
Nigeria Vision 20: 2020 The First National Implementation Plan (2010 – 2013) Volume III: Sectoral Plans and Programmes May 2010 Nigeria Vision 2020 TABLE OF CONTENTS PART V: GOVERNANCE AND GENERAL ADMINISTRATION ................................................................. 2 CHAPTER 1: GOVERNANCE AND POLITICAL SYSTEM CHAPTER 2: JUSTICE AND JUDICIARY ................................................................................................................... 16 CHAPTER 3: LEGISLATURE .................................................................................................................................... 30 CHAPTER 4: ANTI-CORRUPTION ........................................................................................................................... 41 CHAPTER 5: PUBLIC SERVICE ................................................................................................................................ 47 CHAPTER 6: BUSINESS ENVIRONMENT AND COMPETITIVENESS .......................................................................... 81 CHAPTER 7: CORPORATE GOVERNANCE .............................................................................................................. 88 CHAPTER 8: SECURITY AND CIVIL DEFENCE ........................................................................................................ 94 CHAPTER 9: MEDIA AND COMMUNICATION ......................................................................................................... 137 CHAPTER 10: REGIONAL DEVELOPMENT ........................................................................................................... -

NIGERIA: REFERENCE MAP CAMEROON REFUGEE SITUATION Plateau Karim-Lamido Ardo-Kola
| NIGERIA NIGERIA: REFERENCE MAP CAMEROON REFUGEE SITUATION Plateau Karim-Lamido Ardo-Kola H!&M&M Gassol Ibi Nasarawa &M &M &M Wukari Bali &M Guma &M Agatu H! Taraba MakuH!rdi &M &M Logo Apa Ukum Donga Gwer West Tarka &M Gashaka &M Buruku &M Gwer East Gboko Oturkpo Benue Katsina-Ala Kurmi Ohimini Takum ¥&M & &M H! &M Ushongo Ussa Konshisha Okpokwu Ogbadibo Obi Sardauna & Oju Vandeikya Enugu Ado Kwande Bekwara &M ¥ Ikyogem Yala & Ö Donga-Mantung Ukende Ukende Adagom I Ö Ö Adagom ¥ Ikyogem Menchum ¥ Obudu Ogoja Obanliku H! Ebonyi H! Boki Ikom National boundary Obubra &M State boundary Abi Etung &M Manyu Yakurr LGA Boundary Affected Location Abia Cross River Republic of Rivers H! Biase Cameroon H! City Akamkpa Ini &M Town Ö Refugee Settlement Obot Akara Ikono Ibiono Ibom&M Ikot EkH!pene & Itu Odukpani UNHCR Sub Office Essien Udim & Abak H! UNHCR Field Office Uyo Ika Uruan Calabar-¥Municipal Etim Ekpo H!& ¥ Ibesikpo Asutan Current Registration Sites Nsit Ibom Akpabuyo Ukanafun Calabar South Nsit Atai Okobo &M Ndian Oruk Anam Oron Bakassi Etinan Udung Uko Nsit Ubium Mbo Mkpat Enin Urue-Offong/Oruko &M Eket Esit - Eket Mbo Ikot Abasi Onna Mbo Nigeria Overview Map Ibeno The boundaries and names shown and the Eastern Obolo designations used on this map do not imply official endorsement or accepatence by the United Nations. Sources: UNHCR Nigeria, HDX Basemap: Open Street Map ² Creation date: January 2020 Scale: 1:700,000 Km Email: [email protected] 0 600 1,200 2,400 Website: http://www.unchr.©o OrpegnS/treetMap (and) contributors, CC-BY-SA. -

Facts on NNPC/MPN Multi-Year Projects in Akwa Ibom State
The Facts on NNPC/MPN Multi-Year Projects in Akwa Ibom State Background What Special Projects II is not: 1. It is NOT a palliative or compensation for any oil spill In July 2013, Mobil Producing Nigeria Unlimited (MPN), operator of the Nigerian National Petroleum What Special Projects II is: Corporation (NNPC)/MPN Joint Venture, 1. It is a social investment across our neighbouring and coastal announced plans to make multi-year social communities 2. It is an act of goodwill done in good faith investments with a total value of N26 Billion in Akwa 3. Communities undertook to sustain peaceful Ibom State. These investments are in addition to the atmosphere/conduct joint venture's regular annual community Project Governance and Payments: development projects. 1. Managed by a Steering Committee comprising Akwa Ibom The social investment package funds both short- State Government, communities and the JV and long-term projects for MPN's neighbouring 2. Stewarded by indigenous Project Managers communities, as well as other parts of Akwa Ibom 3. Projects and contractors are nominated by benefitting State. Short-term projects include contributions for communities the Eket-Ibeno road reconstruction and Special 4. JV pays project managers on agreed performance milestones Community Projects II in the four communities Update on Activities and Payment Status: surrounding MPN's operations in Qua Iboe Terminal The Special Projects II, since inception in 2014, has provided the (QIT), as well as four coastal communities. following: Community Total Project -

Implementing the Surge Hiv Response in Akwa Ibom an Accelerated Hiv Epidemic Control Drive Content
TECHNICAL BRIEF - 2019 A technical guide for local program local program for guide A technical Ibom State... in Akwa managers IMPLEMENTING THE SURGE HIV RESPONSE IN AKWA IBOM AN ACCELERATED HIV EPIDEMIC CONTROL DRIVE CONTENT 1 Understanding the shape of the HIV epidemic in Akwa Ibom 2 Rationale for the surge HIV response 3 Overaching themes of the surge HIV response 4 Community antiretroviral therapy management (CAM) 5 Preimplementation activities 6 The community antiretroviral therapy management team 7 HIV case finding; the fulcrum point of the surge HIV response 8 Linkage to antiretroviral therapy 9 Viral load services in the context of community ART services 10 Coordination and monitoring of CAM services 11 Conclusion 12 Annex TECHNICAL GUIDANCE TECHNICAL GUIDE PAGE 3 UNDERSTANDING THE SHAPE OF THE HIV EPIDEMIC IN AKWA IBOM STATE Fig 1: HIV prevalence in Nigeria (NAIIS 2018) Nigeria has made progress in the fight against HIV/AIDS. The just concluded national AIDS indicator impact survey (NAIIS) revealed that the national Estimated 13,000 new cases prevalence of HIV is 1.4% with an estimated 1.9 million people infected with per year Adolescents 15-19 years the virus. With a mixed epidemic, only 7 out of the 36 states in the country account for 5000 cases % Incidence among cohabiting partners: 1.60 ---- AKAIS account for over 50% of this burden. The top 3 states by burden include Rivers, Benue and Akwa Ibom states. Akwa Ibom state has the highest prevalence in the country at 5.5% with an estimated burden of 178,000 people living with HIV. -

A Comparative Analysis of the Availability of Information Resources on Ibibio Culture in the University of Uyo and Akwa Ibom State Public Library
Journal of Education and Practice www.iiste.org ISSN 2222-1735 (Paper) ISSN 2222-288X (Online) Vol.6, No.5, 2015 A Comparative Analysis of the Availability of Information Resources on Ibibio Culture in the University Of Uyo And Akwa Ibom State Public Library HENRY ITOHOWO OKON (Ph.D.), JEHU S. SIMON Department of Educational Technology & Library Science, Faculty of Education, University of Uyo, Uyo [email protected] [email protected] INIOBONG AKAI The Library Department, Akwa Ibom State University, Obio Akpa Campus, Akwa Ibom State. [email protected] ABSTRACT This study reports the results of a survey of the available holdings of information resources on Ibibio culture in the University of Uyo Library and Akwa Ibom State Library. The specific objectives of the study were to determine the different size of information resources on funeral, fattening (Mbobo), taboos, myths as well as dissemination in the University of Uyo and Akwa Ibom State Libraries. Survey research design was used for the study. 3,854 information sources in the University of Uyo and Akwa Ibom State Libraries constituted the population of the study, with all estimated 2,276 (100%) forming the sample size of the study. Checklist was used to obtain data for the study. Chi-square was used for the data analysis. The major research findings based on the postulated objectives of the study revealed that there was a significant difference in the information resources available in the University of Uyo Library and Akwa Ibom State Library. The study concluded that libraries must oversee additional problems and increase the acquisition of information resources on Ibibio culture to further cub the dwindling nature of its collection and possible extinction of this information resources. -

Nigeria Conflict Bulletin: Akwa Ibom State
THE FUND FOR PEACE Nigeria Conflict Bulletin: Akwa Ibom State Patterns and Trends, January 2012 - J u n e 2 0 1 5 Overall, between 2012-2015, Akwa This Conflict Bulletin provides a brief incidents and fatalities over time. The bar Ibom was the second most peaceful state in snapshot of the trends and patterns of chart shows the relative trend of incidents the Niger Delta region as measured by conflict risk factors at the State and LGA of insecurity by LGA per capita. reported fatalities per capita. Udom levels, drawing on the data available on the Emmanuel of the People’s Democratic Party P4P Digital Platform for Multi-Stakeholder The summaries draw on data collected by (PDP) won the gubernatorial election in April Engagement (www.p4p-nigerdelta.org). It ACLED, FFP’s UNLocK, the Council on 2015, to replace outgoing Governor represents a compilation of the data from Foreign Relations’ NST, WANEP Nigeria, CSS/ Godswill Akpabio (also PDP) who was the sources listed below, not necessarily the ETH Zurich, NEEWS2015, and Nigeria Watch elected in 2007. Election violence was opinions of FFP or any other organization integrated on the P4P platform. They also reported in both 2011 and 2015. After the that collaborated on the production of this draw on data and information from most recent election, supporters of the bulletin. “Violence in Nigeria: Patterns and Trends,” opposition All Progressives Congress (APC) by Patricia Taft and Nate Haken (Springer protested the results, alleging irregularities. The screenshot of the heat map on this Press, April 2015). page shows the relative distribution of Other issues reported in the last three and a incidents from one LGA to the next from half years include land conflict and January 2012 to June 2015. -

Mobil Producing Nigeria Commissions New Office Extension in Akwa Ibom State
A Publication of ExxonMobil Subsidiaries in Nigeria ISSN 1597 - 0442 Mobil Producing Nigeria Commissions New Office Extension in Akwa Ibom State e 2012 d Reagan i 2 MON 2012 Memorial s Annual General “Project New Lab n Meeting Care” from Esso I EDITION BUSINESS COMMUNITY RELATIONS Governor Akpabio Esso handover a new commissions multi-billion Health Care Facility to naira MPN Office Extension Nanka Community 2 22 BUSINESS COMMUNITY RELATIONS EM affiliates launch Upstream Nigeria donates a new engagement charity walk proceeds to initiative PH Cancer Centre 11 30 SHE EMPLOYEE Upstream Nigeria receives Inoyo Havens high scores on Emergency Response Drills 19 33 ExxonMobil News ISSN: 1597 0442 Esso Exploration and Production Nigeria Limited Mobil Oil Nigeria plc Mobil Producing Nigeria Unlimited Edition 2, 2012 Chairman/Managing Director. EEPNL & MPN and Lead Country Manager: Mark R. Ward Chairman/Managing Director, MON: Adetunji A. Oyebanji Director, Lubricants & Specialties, MON: A. J. MacNaughton General Manager, Public & Government Affairs: Paul C. Arinze Editors: Nigel Cookey-Gam, Akin Fatunke Editorial Team: Adeyemi Fakayejo, Ozemoya Okordion, Ernest Omo-Ojo, Cornell Udeagwu, Patrick Utomi Editorial Support: Peniel Media Ltd. Cover/Concept/Graphic Designs: Taiwo Ogunbiyi Registered Office: Mobil House, 1, Lekki Expressway, P.M.B. 12054, Victoria Island, Lagos. Published by Public & Government Affairs Department for Employees of ExxonMobil subsidiaries in Nigeria. All correspondence to: The Editor, Public & Government Affairs Department, Mobil Producing Nigeria Unlimited, Mobil House, 1, Lekki Expressway, P.M.B. 12054, Victoria Island, Lagos. Tel: 01-2621640, 2621660. All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, or stored in any retrieval system of any nature, without the written permission of the editors. -

Seasonal Variation of Meteorological Factors on Air Parameters and the Impact of Gas Flaring on Air Quality of Some Cities in Niger Delta (Ibeno and Its Environs)
Vol. 9(3), pp. 218-227, March 2015 DOI: 10.5897/AJEST2015.1867 Article Number: 018C04950850 African Journal of Environmental Science and ISSN 1996-0786 Copyright © 2015 Technology Author(s) retain the copyright of this article http://www.academicjournals.org/AJEST Full Length Research Paper Seasonal variation of meteorological factors on air parameters and the impact of gas flaring on air quality of some cities in Niger Delta (Ibeno and its environs) Asuoha, Adaku N.1 and Osu Charles I.2* 1Safety Health and Environmental Department, Mobil Producing Nigeria, Nigeria. 2Department of Pure and Industrial Chemistry, University of Port Harcourt, P.M.B 5323 Choba, Port Harcourt, Rivers State, Nigeria. Received 19 January, 2015, Accepted 29 January, 2015 The impacts of gas flaring on meteorological factors at Ibeno, Eket, Onna, Esit Eket and Umudike - Nigeria were investigated by measuring air quality parameters. The results show that the mean concentration of air parameters value were below Federal Environmental Protection Agency (FEPA) and United States Environmental Protection Agency (USEPA) National air quality standards with exception of carbon monoxide which exceeded the limit of 35 ppm in March at Ibeno. Concentration of air parameters at Umudike showed a similar trend to that of study locations at Eket, Ibeno, Esit Eket and - Onna. Air quality parameters (Cl , SPM and SO2) were found to have positive correlation with vapour pressure, humidity and rainfall values in the study areas. It was also established that a positive correlation exits between NO2, H2S, SO2, SPM, chloride, carbon monoxide and wind speed relative humidity, temperature and vapour pressure in the study locations.