Clam Tissue Collection Report Wyckoff/Eagle Harbor Superfund Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Download Download
Appendix C: An Analysis of Three Shellfish Assemblages from Tsʼishaa, Site DfSi-16 (204T), Benson Island, Pacific Rim National Park Reserve of Canada by Ian D. Sumpter Cultural Resource Services, Western Canada Service Centre, Parks Canada Agency, Victoria, B.C. Introduction column sampling, plus a second shell data collect- ing method, hand-collection/screen sampling, were This report describes and analyzes marine shellfish used to recover seven shellfish data sets for investi- recovered from three archaeological excavation gating the siteʼs invertebrate materials. The analysis units at the Tseshaht village of Tsʼishaa (DfSi-16). reported here focuses on three column assemblages The mollusc materials were collected from two collected by the researcher during the 1999 (Unit different areas investigated in 1999 and 2001. The S14–16/W25–27) and 2001 (Units S56–57/W50– source areas are located within the village proper 52, S62–64/W62–64) excavations only. and on an elevated landform positioned behind the village. The two areas contain stratified cultural Procedures and Methods of Quantification and deposits dating to the late and middle Holocene Identification periods, respectively. With an emphasis on mollusc species identifica- The primary purpose of collecting and examining tion and quantification, this preliminary analysis the Tsʼishaa shellfish remains was to sample, iden- examines discarded shellfood remains that were tify, and quantify the marine invertebrate species collected and processed by the site occupants for each major stratigraphic layer. Sets of quantita- for approximately 5,000 years. The data, when tive information were compiled through out the reviewed together with the recovered vertebrate analysis in order to accomplish these objectives. -

A Review of the Biology and Fisheries of Horse Clams (Tresus Capax and Tresus Nuttallii)
Fisheries and Oceans Pêches at Océans Canada Canad a Canadian Stock Assessment Secretariat Secrétariat canadien pour l'évaluation des stocks Research Document 98/8 8 Document de recherche 98/8 8 Not to be cited without Ne pas citer sans permission of the authors ' autorisation des auteurs ' A Review of the Biology and Fisheries of Horse Clams (Tresus capax and Tresus nuttallii) R. B . Lauzier, C . M. Hand, A. Campbell and S .Heizerz Fisheries and Oceans Canada Pacific Biological Station, Stock Assessment Division, Nanaimo, B.C. V9R 5K6 2 Fisheries and Oceans Canada South Coast Division, N anaimo, B.C. V9T 1K3 ' This series documents the scientific basis for the ' La présente série documente les bases scientifiques evaluation of fisheries resources in Canada . As des évaluations des ressources halieutiques du such, it addresses the issues of the day in the time Canada. Elle traite des problèmes courants selon les frames required and the documents it contains are échéanciers dictés. Les documents qu'elle contient not intended as definitive statements on the subjects ne doivent pas être considérés comme des énoncés addressed but rather as progress reports on ongoing définitifs sur les sujets traités, mais plutôt comme investigations . des rapports d'étape sur les études en cours . Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé Secretariat. au secrétariat . ISSN 1480-4883 Ottawa, 199 8 Canada* Abstract A review of the biology and distribution of horse clams (Tresus capax and Tresus nuttallii)and a review of the fisheries of horse clams from British Columbia, Washington and Oregon is presented, based on previous surveys, scientific literature, and technical reports . -

Monda Y , March 22, 2021
NATIONAL SHELLFISHERIES ASSOCIATION Program and Abstracts of the 113th Annual Meeting March 22 − 25, 2021 Global Edition @ http://shellfish21.com Follow on Social Media: #shellfish21 NSA 113th ANNUAL MEETING (virtual) National Shellfisheries Association March 22—March 25, 2021 MONDAY, MARCH 22, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound Chesapeake Bay Monterey Bay SHELLFISH ONE HEALTH: SHELLFISH AQUACULTURE EPIGENOMES & 8:30-10:30 AM CEPHALOPODS OYSTER I RESTORATION & BUSINESS & MICROBIOMES: FROM SOIL CONSERVATION ECONOMICS TO PEOPLE WORKSHOP 10:30-10:45 AM MORNING BREAK THE SEA GRANT SHELLFISH ONE HEALTH: EPIGENOMES COVID-19 RESPONSE GENERAL 10:45-1:00 PM OYSTER I RESTORATION & & MICROBIOMES: FROM SOIL TO THE NEEDS OF THE CONTRIBUTED I CONSERVATION TO PEOPLE WORKSHOP SHELLFISH INDUSTRY 1:00-1:30 PM LUNCH BREAK WITH SPONSOR & TRADESHOW PRESENTATIONS PLENARY LECTURE: Roger Mann (Virginia Institute of Marine Science, USA) (LIVE) 1:30-2:30 PM Chesapeake Bay EASTERN OYSTER SHELLFISH ONE HEALTH: EPIGENOMES 2:30-3:45 PM GENOME CONSORTIUM BLUE CRABS VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP CONSERVATION TO PEOPLE WORKSHOP BLUE CRAB GENOMICS EASTERN OYSTER & TRANSCRIPTOMICS: SHELLFISH ONE HEALTH: EPIGENOMES 3:45–5:45 PM GENOME CONSORTIUM THE PROGRAM OF THE VIBRIO RESTORATION & & MICROBIOMES: FROM SOIL WORKSHOP BLUE CRAB GENOME CONSERVATION TO PEOPLE WORKSHOP PROJECT TUESDAY, MARCH 23, 2021 DAILY MEETING UPDATE (LIVE) 8:00 AM Gulf of Maine Gulf of Maine Gulf of Mexico Puget Sound -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Spatial Comparison of Bay Clam Morphometrics and Age Structure in Three Oregon Estuaries
Spatial comparison of bay clam morphometrics and age structure in three Oregon estuaries Cinamon Moffett, Anthony D’Andrea, and Justin Ainsworth Age-Length Keys Introduction Oregon Department of Fish and Wildlife, Marine Resources Program, Newport, OR • Age-length keys were created using the Historical Creel data. Management of Oregon’s bay clam resources and habitats are the [email protected] • Keys were then applied to the Recent Creel and SEACOR data. responsibility of the Oregon Department of Fish and Wildlife (ODFW) Data Set Years Sampled Data Type Methods Parameters • The differences in mean age between data sets were evaluated using Shellfish Program. Bay clams, which are referred to and managed Data Sets Historical Creel 1983-1991 fisheries dependent recreational length, age from a KS-test (α=0.05). Each estuary with a blue arrow has collectively, include: butter clams (Saxidomus gigantea), cockles harvester growth checks This analysis utilized four interviews significant differences in their estimated age from the known age (Clinocardium nuttallii), gaper clams (Tresus capax), and native existing data sets, each with Recent Creel 2008-2014 fisheries dependent recreational length Do bay clam structure. littleneck clams (Leukoma staminea). This analysis examined bay clam unique characteristics. harvester morphometrics or Most estimated age morphometrics and age structures in three recreationally important interviews age structures Example Age- structures are Oregon estuaries: Netarts, Tillamook and Yaquina bays. Presented here SEACOR-full Netarts-2013-2014 fisheries independent stratified random length, weight, vary on a spatial Length Key significantly different (subtidal and intertidal) Tillamook-2010-2012 sampling width, density between estuaries and are the results from gapers and cockles, which differ in several key life Yaquina-2012 scale? between data sets history traits. -

Review of the Literature on Bivalve Cytogenetics in the Last Ten Years
Cah. Biol. Mar. (2002) 43 : 17-26 Review of the literature on bivalve cytogenetics in the last ten years Catherine THIRIOT-QUIEVREUX Observatoire Océanologique, Université P. et M. Curie – CNRS - INSU, BP 28, 06230 Villefranche-sur-Mer, France Fax: (33) 4 93 76 38 48; E-mail: [email protected] Abstract: This paper provides a review of the studies on bivalve chromosomes since 1992, in order to gather available data and to highlight the recent progress in different fields of cytogenetics: karyotype and chromosome markers, genome size, aneuploidy, natural and induced polyploidy, and hybridization. Résumé: Revue des travaux des dix dernières années sur l’étude cytogénétique des bivalves. Cet article présente une revue sur l’étude des chromosomes des bivalves depuis 1992 afin de rassembler les données disponibles et de souligner les pro- grès récents dans les différents domaines de la cytogénétique : caryotype et marqueurs chromosomiques, taille du génome, aneuploïdie, polyploïdie naturelle et induite, et hydridisation. Keywords: Bivalvia, Chromosomes, Cytogenetics Introduction review, 1985). Later, the development of banding techniques which allowed chromosome identification in Cytogenetic studies encompass different levels of biological karyotypes began to be applied in bivalves (see Thiriot- organization ranging from the morphological to the Quiévreux review, 1994). Since these reviews, the study of molecular, depending on the applicable technology. bivalve chromosomes has greatly progressed in Chromosomes can be studied as a morphological karyological as well as molecular information, as a result of manifestation of the genome in terms of their routine application of several banding techniques and the microscopically visible size, shape, number and behaviour development of techniques for in situ hybridization. -

Molluscs: Bivalvia Laura A
I Molluscs: Bivalvia Laura A. Brink The bivalves (also known as lamellibranchs or pelecypods) include such groups as the clams, mussels, scallops, and oysters. The class Bivalvia is one of the largest groups of invertebrates on the Pacific Northwest coast, with well over 150 species encompassing nine orders and 42 families (Table 1).Despite the fact that this class of mollusc is well represented in the Pacific Northwest, the larvae of only a few species have been identified and described in the scientific literature. The larvae of only 15 of the more common bivalves are described in this chapter. Six of these are introductions from the East Coast. There has been quite a bit of work aimed at rearing West Coast bivalve larvae in the lab, but this has lead to few larval descriptions. Reproduction and Development Most marine bivalves, like many marine invertebrates, are broadcast spawners (e.g., Crassostrea gigas, Macoma balthica, and Mya arenaria,); the males expel sperm into the seawater while females expel their eggs (Fig. 1).Fertilization of an egg by a sperm occurs within the water column. In some species, fertilization occurs within the female, with the zygotes then text continues on page 134 Fig. I. Generalized life cycle of marine bivalves (not to scale). 130 Identification Guide to Larval Marine Invertebrates ofthe Pacific Northwest Table 1. Species in the class Bivalvia from the Pacific Northwest (local species list from Kozloff, 1996). Species in bold indicate larvae described in this chapter. Order, Family Species Life References for Larval Descriptions History1 Nuculoida Nuculidae Nucula tenuis Acila castrensis FSP Strathmann, 1987; Zardus and Morse, 1998 Nuculanidae Nuculana harnata Nuculana rninuta Nuculana cellutita Yoldiidae Yoldia arnygdalea Yoldia scissurata Yoldia thraciaeforrnis Hutchings and Haedrich, 1984 Yoldia rnyalis Solemyoida Solemyidae Solemya reidi FSP Gustafson and Reid. -

Reproductive Ecology and Dispersal Potential of Varnish Clam Nuttallia Obscurata, a Recent Invader in the Northeast Pacific Ocean
MARINE ECOLOGY PROGRESS SERIES Vol. 320: 195–205, 2006 Published August 29 Mar Ecol Prog Ser Reproductive ecology and dispersal potential of varnish clam Nuttallia obscurata, a recent invader in the Northeast Pacific Ocean Sarah E. Dudas1, 3,*, John F. Dower1, 2 1Department of Biology, and 2School of Earth & Ocean Sciences, University of Victoria, PO Box 3020 STN CSC, Victoria, British Columbia V8W 3N5, Canada 3Present address: Department of Zoology, Oregon State University, 3029 Cordley Hall, Corvallis, Oregon 97331, USA ABSTRACT: The fecundity, larval development, and temperature and salinity tolerances were deter- mined for the varnish clam Nuttallia obscurata (Reeve 1857), a recently introduced species in the Northeast Pacific. Adult varnish clams from 2 populations were collected in British Columbia, Canada throughout the spawning season to determine sex, fecundity, and timing of spawning. Adult varnish clams were also spawned in the laboratory and the larvae reared at a range of temperatures and salinities. The highest larval growth rates were observed in the 20°C and 20 psu treatments. Planktonic duration ranged from 3 to potentially 8 wk, with higher temperatures and salinities result- ing in a shorter planktonic phase. Larvae reared at 9°C, and at 10 and 15 psu, grew slowly and sur- vived for a minimum of 1 mo but did not reach metamorphosis. These results indicate that varnish clam larvae have a wide range of salinity and temperature tolerances, but grow optimally at warmer temperatures and higher salinities. Varnish clams have comparable larval environmental tolerances and spawning duration to co-occurring bivalves. However, their fecundity appears to be slightly higher and they reach sexual maturity earlier, potentially providing an advantage in establishing new populations. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
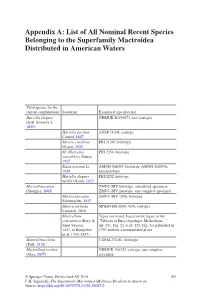
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste