CT Imaging of Peritoneal Carcinomatosis and Its Mimics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

BODY CAVITIES and MESENTERY
73: BODY CAVITIES and MESENTERY We've already mentioned that all the organs in the body are wrapped in "bags" made of thin layers of connective tissue. These bags are often inside of other bags, or even inside of several bags. The largest bags define areas that we call body cavities. There are three main cavities: the thoracic cavity, the abdominal cavity and the pelvic cavity. The thoracic cavity is subdivided into three smaller cavities: the pleural cavity (containing the lungs), the mediastinum(in the middle), and the pericardial cavity (containing the heart). The pleural cavity is easy to understand because it simply contains the lungs. The pericardial cavity contains not only the heart itself, but the large blood vessels that come out of it, such as the aorta. The pericardial cavity is inside of the third cavity, the mediastinum. ("Media" means "middle" and "stinum" can refer to the "sternum," which is the bone that runs down the center of the ribcage.) The mediastinum contains not only the pericardial cavity but also part of the esophagus and trachea, the thymus (remember this organ from module 2 on the immune system?), and quite a few nerves and lymph nodes. The thin layers of connective tissues that surround these cavities are made primarily of collagen and elastin (produced by fibroblast cells) but they also contain some very tiny nerves and blood vessels, as well as cells that make serous fluid. As we've seen in the past few lessons, the diaphragm separates the thoracic cavity from the abdominal cavity. The abdominal cavity contains the stomach, the spleen, the tail of the pancreas, the last half of the duodenum, the small intestines, most of the large intestines, and the mesentery (thin layers of connective tissue that anchor the intestines to the back wall of the abdominal cavity). -
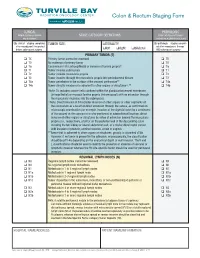
Colon & Rectum Staging Form
Colon & Rectum Staging Form CLINICAL PATHOLOGIC Extent of disease before STAGE CATEGORY DEFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical – staging completed TUMOR SIZE: LATERALITY: y pathologic – staging complet- after neoadjuvant therapy but ed after neoadjuvant therapy before subsequent surgery left right bilateral AND subsequent surgery PRIMARY TUMOR (T) TX Primary tumor cannot be assessed TX T0 No evidence of primary tumor T0 Tis Carcinoma in situ: intraepithelial or invasion of lamina propria* Tis T1 Tumor invades submucosa T1 T2 Tumor invades muscularis propria T2 T3 Tumor invades through the muscularis propria into pericolorectal tissues T3 T4a Tumor penetrates to the surface of the visceral peritoneum** T4a T4b Tumor directly invades or is adherent to other organs or structures^,** T4b *Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa. ^Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (for example, invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retro-peritoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix or vagina). **Tumor that is adherent to other organs or structures, grossly, is classified cT4b. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Development of the Serosal Mesothelium
J. Dev. Biol. 2013, 1, 64-81; doi:10.3390/jdb1020064 OPEN ACCESS Journal of Developmental Biology ISSN 2221-3759 www.mdpi.com/journal/jdb Review Development of the Serosal Mesothelium Nichelle I. Winters and David M. Bader * Department of Medicine, Vanderbilt University, 2220 Pierce Ave Nashville, TN 37232, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-615-936-1976; Fax: +1-615-936-3527. Received: 3 May 2013; in revised form: 13 June 2013 / Accepted: 19 June 2013 / Published: 26 June 2013 Abstract: Mesothelia in the adult vertebrate are the simple squamous epithelia covering all coelomic organs and body cavities. Until recently, analysis of the generation and differentiative potential of mesothelia in organogenesis has largely focused on development of visceral mesothelium of the heart; the epicardium and its progenitor, the proepicardium. Here, we review emerging data on the development and differentiation of serosal mesothelium, the covering of the gastrointestinal tract. This literature demonstrates that serosal mesothelium is generated through a completely different mechanism than that seen in the heart suggesting that commitment of progenitors to this cell lineage does not follow a common pathway. The differentiative potential of serosal mesothelium is also discussed in comparison to that observed for progeny of the proepicardium/epicardium. In our review of the literature, we point out gaps in our understanding of serosal mesothelial development and that of mesothelial development as a whole. Keywords: mesothelium; proepicardium; epicardium; intestine; heart 1. Mesothelia: Broad Definition Mesothelia are simple squamous epithelia that line coelomic cavities and organs and form the mesenteries. -

TUMOR and TUMOR-LIKE CONDITIONS of the PERITONEUM and OMENTUM/MESENTERY 40Th
TUMOR AND TUMOR-LIKE CONDITIONS OF THE PERITONEUM AND OMENTUM/MESENTERY 40th. Annual Meeting SCBTMR September 9-13, 2017, Nashville, Tennessee Isaac R Francis University of Michigan Department of Radiology University of Michigan Disclosures • None • Acknowledgement: Dr. Ashish Wasnik, M.B.:B.S. Assistant Professor of Radiology University of Michigan PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Rare aggressive tumor • More common in men • Approx. 15% arise from peritoneum • Etiology: Exposure to asbestosis; other causes include mineral fiber exposure, radiation and chronic irritation • Three main histological subtypes: epithelioid (most common), sarcomatous (worst prognosis), and biphasic (mixed) • Frequent mutation of BRCA-Associated Protein (BAP)1 Levy AD et al Radiographics 2008; Alexander HR et al UptoDate 2016 PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Can present as focal, large or confluent peritoneal based mass/es (“dry” type) • Peritoneal nodular or diffuse thickening with ascites (“wet” type) • May show scalloping on adjacent abdominal organs- liver, spleen etc. • Calcification including calcified plaques is uncommon • Can infiltrate bowel mesentery, result in bowel wall thickening and may lead to bowel obstruction Levy AD et al Radiographics 2008 Peritoneal mesothelioma “Dry type” “Wet type” PRIMARY PERITONEAL TUMORS Malignant Mesothelioma: • Cannot be easily distinguished from abdominal carcinomatosis • Absence of a known primary, absence of metastases to other organs or enlarged lymph nodes may help to distinguish -

Mvdr. Natália Hvizdošová, Phd. Mudr. Zuzana Kováčová
MVDr. Natália Hvizdošová, PhD. MUDr. Zuzana Kováčová ABDOMEN Borders outer: xiphoid process, costal arch, Th12 iliac crest, anterior superior iliac spine (ASIS), inguinal lig., mons pubis internal: diaphragm (on the right side extends to the 4th intercostal space, on the left side extends to the 5th intercostal space) plane through terminal line Abdominal regions superior - epigastrium (regions: epigastric, hypochondriac left and right) middle - mesogastrium (regions: umbilical, lateral left and right) inferior - hypogastrium (regions: pubic, inguinal left and right) ABDOMINAL WALL Orientation lines xiphisternal line – Th8 subcostal line – L3 bispinal line (transtubercular) – L5 Clinically important lines transpyloric line – L1 (pylorus, duodenal bulb, fundus of gallbladder, superior mesenteric a., cisterna chyli, hilum of kidney, lower border of spinal cord) transumbilical line – L4 Bones Lumbar vertebrae (5): body vertebral arch – lamina of arch, pedicle of arch, superior and inferior vertebral notch – intervertebral foramen vertebral foramen spinous process superior articular process – mammillary process inferior articular process costal process – accessory process Sacrum base of sacrum – promontory, superior articular process lateral part – wing, auricular surface, sacral tuberosity pelvic surface – transverse lines (ridges), anterior sacral foramina dorsal surface – median, intermediate, lateral sacral crest, posterior sacral foramina, sacral horn, sacral canal, sacral hiatus apex of the sacrum Coccyx coccygeal horn Layers of the abdominal wall 1. SKIN 2. SUBCUTANEOUS TISSUE + SUPERFICIAL FASCIAS + SUPRAFASCIAL STRUCTURES Superficial fascias: Camper´s fascia (fatty layer) – downward becomes dartos m. Scarpa´s fascia (membranous layer) – downward becomes superficial perineal fascia of Colles´) dartos m. + Colles´ fascia = tunica dartos Suprafascial structures: Arteries and veins: cutaneous brr. of posterior intercostal a. and v., and musculophrenic a. -

Abdominal Foregut & Peritoneum Development
Abdominal Foregut & Peritoneum Development - Transverse View Gastrointestinal System > Embryology > Embryology Key Concepts • The peritoneum is a continuous serous membrane that covers the abdominal wall and viscera. - Parietal layer of the peritoneum lines the body wall - Visceral layer envelops the viscera, aka, the organs - In some places, the visceral layer extends from the organs as folds that form ligaments, omenta, and mesenteries. • Viscera is categorized by their relationship to the peritoneum: - Intraperitoneal organs are covered by visceral peritoneum; as we'll see, the stomach is an example of this. - Retroperitoneal organs lie between the body wall and the parietal peritoneum (the kidneys, for example, are retroperitoneal). - Some organs are said to be secondarily retroperitoneal, because during their early embryologic stages, they are enveloped in visceral peritoneum, but later fuse to the body wall and are covered only by parietal peritoneum. Week 5 Just prior to rotation of the stomach. Diagram instructions: draw the outer surface and body wall, and indicate that the body wall is lined by parietal peritoneum. • Organs at the midline, from dorsal to ventral: - Abdominal aorta - Stomach; branches of the right and left vagus nerves extend along the sides of the stomach - Liver • Peritoneal coverings and ligaments: - Dorsal mesogastrium anchors the stomach to the posterior body wall - Visceral peritoneum covers the stomach - Ventral mesogastrium connects the stomach to the liver - Falciform ligament anchors the liver to the ventral body wall As you may recall, the dorsal mesogastrium is a portion of the dorsal mesentery, and the ventral mesogastrium and falciform ligament arose from the ventral mesentery. It's helpful to remember that "gastric," as in mesogastrium, = refers 1 / 2 to the stomach. -

Abdomen Abdomen
Abdomen Abdomen The abdomen is the part of the trunk between the thorax and the pelvis. It is a flexible, dynamic container, housing most of the organs of the alimentary system and part of the urogenital system. The abdomen consists of: • abdominal walls • abdominal cavity • abdominal viscera ABDOMINAL WALL Boundaries: • Superior : - xiphoid proc. - costal arch - XII rib • Inferior : - pubic symphysis - inguinal groove - iliac crest • Lateral: - posterior axillary line ABDOMINAL WALL The regional system divides the abdomen based on: • the subcostal plane – linea bicostalis: between Х-th ribs • the transtubercular plane – linea bispinalis: between ASIS. Epigastrium Mesogastrium Hypogastrium ABDOMINAL WALL The right and left midclavicular lines subdivide it into: Epigastrium: • Epigastric region • Right hypochondric region • Left hypochondric region Mesogastrium: • Umbilical region • Regio lateralis dex. • Regio lateralis sin. Hypogastrium: • Pubic region • Right inguinal region • Left inguinal region Organization of the layers Skin Subcutaneous tissue superficial fatty layer - Camper's fascia deep membranous layer - Scarpa's fascia Muscles Transversalis fascia Extraperitoneal fat Parietal peritoneum Organization of the layers Skin Subcutaneous tissue superficial fatty layer - Camper's fascia deep membranous layer - Scarpa's fascia Muscles Transversalis fascia Extraperitoneal fat Parietal peritoneum Superficial structures Arteries: • Superficial epigastric a. • Superficial circumflex iliac a. • External pudendal a. Superficial structures Veins: In the upper abdomen: - Thoracoepigastric v. In the lower abdomen: - Superficial epigastric v. - Superficial circumflex iliac v. - External pudendal v. Around the umbilicus: - Parumbilical veins • Deep veins: - Intercostal vv. - Superior epigastric v. - Inferior epigastric v. Superficial structures Veins: In the upper abdomen: - Thoracoepigastric v. In the lower abdomen: - Superficial epigastric v. - Superficial circumflex iliac v. - External pudendal v. -

Carcinoma of the Small Intestine Staging Diagram
CARCINOMA OF THE SMALL INTESTINE STAGING DIAGRAM SITE: Duodenum (C17.0) Ileum (C17.2) (excludes ileocaecal valve C 18.0) Jejunum (C17.1) Small Intestine, NOS (C17.9) HISTOLOGY: New Recurrent Disease Referred for Follow up Definitive treatment already received. Previously treated and followed Referred as part of definitive treatment Referred at recurrence. elsewhere before referral. (initial treatment of disease). Staged at initial diagnosis. Staged at initial diagnosis. TNM 2009 T X 0 is 1 1a 1b 2 3 4 Clinical N X 0 1 2 3 M 0 1 TNM 2009 T X 0 is 1 1a 1b 2 3 4 Pathological N X 0 1 2 M 1 Completed by: Date: (dd/mm/yy) Diagnosis/Stage Amended to: Reason: By: Date: (dd/mm/yy) NOTIFY DATA QUALITY & REGISTRY IF STAGE/DIAGNOSIS IS AMENDED FORM #TH-90 Revised August 2010 Photocopy Form as Required CARCINOMA OF THE SMALL INTESTINE STAGING DIAGRAM AJCC 7th Edition for Diagnosis Date > 01 January 2010 Definitions for T, N, and M Descriptors PRIMARY TUMOUR (T)1 TX Primary tumour cannot be assessed T0 No evidence of primary tumour Tis Carcinoma in situ T1 Tumour invades lamina propria, muscularis mucosae or submucosa T1a Tumour invades lamina propria or muscularis mucosae T1b Tumour invades submucosa T2 Tumour invades muscularis propria T3 Tumour invades subserosa or non-peritonealized perimuscular tissue (mesentery or retroperitoneum2) with extension 2 cm or less T4 Tumour perforates visceral peritoneum or directly invades other organs or structures (includes other loops of small intestine, mesentery, or retroperitoneum more than 2 cm and abdominal wall by way of serosa; for duodenum only, invasion of pancreas) Note1: This classification does not apply to carcinomas of the ampulla of Vater (see Pancreas/Ampulla of Vater staging diagram). -

GI Tract Anatomy
SHRI Video Training Series 2018 dx and forward Recorded 1/2020 Colorectal Introduction & Anatomy Presented by Lori Somers, RN 1 Iowa Cancer Registry 2020 Mouth GI Tract Pharynx Anatomy Esophagus Diaphragm Liver Stomach Gallbladder Pancreas Large Small Intestine Intestine (COLON) 2 Anal Canal 1 Colorectal Anatomy Primary Site ICD-O Codes for Colon and Rectum Transverse Hep. Flex C18.4 Splen. Flex C18.3 C18.5 Ascending Large Descending C18.2 Intestine, C18.6 NOS C18.9 Cecum C18.0 Sigmoid C18.7 Appendix C18.1 Rectum C20.9 Rectosigmoid C19.9 3 ILEOCECAL JUNCTION ILEUM Ileocecal sphincter Opening of appendix APPENDIX 4 2 Rectum, Rectosigmoid and Anus Rectosigmoid junction Sigmoid colon Peritoneal Rectum reflection Dentate line Anus 5 Anal verge Peritoneum: serous membrane lining the interior of the abdominal cavity and covers the abdominal organs. Rectum is “extraperitoneal” Rectum lies below the peritoneal reflection and outside of peritoneal cavity 6 3 Greater Omentum Liver Stomach Vessels Ligament Greater Omentum: (reflected upward) Gallbladder Greater Greater Omentum Omentum Transverse colon coils of jejunum Ascending Descending colon colon Appendix Cecum coils of ileum slide 9 slide 10 7 Mesentery (Mesenteries): folds of peritoneum- these attach the colon to the posterior abdominal wall. Visceral peritoneum: = Serosa covering of colon (organs) Parietal peritoneum: = Serosa covering of ABD cavity (body cavities) 8 4 Colon & Rectum Wall Anatomy Lumen Mucosa Submucosa Muscularis propria Subserosa Serosa Peritoneum 9 Layers of Colon Wall -

Cystic Lymphangioma Involving the Mesentery and the Retroperitoneum: a Case Report1
J Korean Radiol Soc 2005;52:347-350 Cystic Lymphangioma Involving the Mesentery and the Retroperitoneum: A Case Report1 Dong Hun Kim, M.D.1, 2, Joo Nam Byun, M.D., Ji-Youn Jang, M.D.2 Cystic lymphangioma is uncommon angiomatous tumor that mainly occurs in the neck. Less than 1% of these tumors affect the mesentery, retroperitoneum and greater omentum. In particular, the cystic lymphangioma involving the mesentery and the retroperitoneum is a rare lesion. We report here on an uncommon case of cystic lym- phangioma that presented as a multilocular mass involving the mesentery and the retroperitoneum, and we also present a brief review of the relevant literature. Index words : Mesentery, neoplasms Retroperitoneum, neoplasms Cysts, CT Lymphangioma Cystic lymphangiomas involving the mesentery and ment of general surgery for an operation on an intraab- the retroperitoneum are rare tumors. Most of the cystic dominal mass that was incidentally detected. He had lymphangiomas occur in the neck, and they generally back pain after minor trauma 4 weeks earlier, and mag- have a single cavity with only only a small proportion of netic resonance imaging (MRI) demonstrated a huge these tumor being multilocular. The cystic spaces are cystic mass extending from the upper intraabdominal lined with a single layer of endothelium and there are cavity to the pelvic cavity, and there was herniation of small lymphoid aggregates in the cyst’s wall that help the nucleus pulposus at the L4-5 level (Fig. 1A). The pa- distinguish lymphangiomas from the simple cysts of the tient’s past medical history was unremarkable and he mesentery.