Baseline Acidity of Percipitation at the South Pole During the Last Two Millennia J H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Office of Polar Programs
DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA COMPREHENSIVE ENVIRONMENTAL EVALUATION DRAFT (15 January 2004) FINAL (30 August 2004) National Science Foundation 4201 Wilson Boulevard Arlington, Virginia 22230 DEVELOPMENT AND IMPLEMENTATION OF SURFACE TRAVERSE CAPABILITIES IN ANTARCTICA FINAL COMPREHENSIVE ENVIRONMENTAL EVALUATION TABLE OF CONTENTS 1.0 INTRODUCTION....................................................................................................................1-1 1.1 Purpose.......................................................................................................................................1-1 1.2 Comprehensive Environmental Evaluation (CEE) Process .......................................................1-1 1.3 Document Organization .............................................................................................................1-2 2.0 BACKGROUND OF SURFACE TRAVERSES IN ANTARCTICA..................................2-1 2.1 Introduction ................................................................................................................................2-1 2.2 Re-supply Traverses...................................................................................................................2-1 2.3 Scientific Traverses and Surface-Based Surveys .......................................................................2-5 3.0 ALTERNATIVES ....................................................................................................................3-1 -
Texts G7 Sout Pole Expeditions
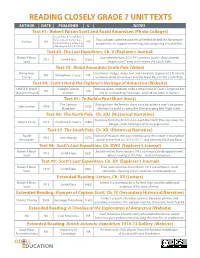
READING CLOSELY GRADE 7 UNIT TEXTS AUTHOR DATE PUBLISHER L NOTES Text #1: Robert Falcon Scott and Roald Amundsen (Photo Collages) Scott Polar Research Inst., University of Cambridge - Two collages combine pictures of the British and the Norwegian Various NA NA National Library of Norway expeditions, to support examining and comparing visual details. - Norwegian Polar Institute Text #2: The Last Expedition, Ch. V (Explorers Journal) Robert Falcon Journal entry from 2/2/1911 presents Scott’s almost poetic 1913 Smith Elder 1160L Scott “impressions” early in his trip to the South Pole. Text #3: Roald Amundsen South Pole (Video) Viking River Combines images, maps, text and narration, to present a historical NA Viking River Cruises NA Cruises narrative about Amundsen and the Great Race to the South Pole. Text #4: Scott’s Hut & the Explorer’s Heritage of Antarctica (Website) UNESCO World Google Cultural Website allows students to do a virtual tour of Scott’s Antarctic hut NA NA Wonders Project Institute and its surrounding landscape, and links to other resources. Text #5: To Build a Fire (Short Story) The Century Excerpt from the famous short story describes a man’s desperate Jack London 1908 920L Magazine attempts to build a saving =re after plunging into frigid water. Text #6: The North Pole, Ch. XXI (Historical Narrative) Narrative from the =rst man to reach the North Pole describes the Robert Peary 1910 Frederick A. Stokes 1380L dangers and challenges of Arctic exploration. Text #7: The South Pole, Ch. XII (Historical Narrative) Roald Narrative recounts the days leading up to Amundsen’s triumphant 1912 John Murray 1070L Amundsen arrival at the Pole on 12/14/1911 – and winning the Great Race. -

Federal Register/Vol. 84, No. 78/Tuesday, April 23, 2019/Rules
Federal Register / Vol. 84, No. 78 / Tuesday, April 23, 2019 / Rules and Regulations 16791 U.S.C. 3501 et seq., nor does it require Agricultural commodities, Pesticides SUPPLEMENTARY INFORMATION: The any special considerations under and pests, Reporting and recordkeeping Antarctic Conservation Act of 1978, as Executive Order 12898, entitled requirements. amended (‘‘ACA’’) (16 U.S.C. 2401, et ‘‘Federal Actions to Address Dated: April 12, 2019. seq.) implements the Protocol on Environmental Justice in Minority Environmental Protection to the Richard P. Keigwin, Jr., Populations and Low-Income Antarctic Treaty (‘‘the Protocol’’). Populations’’ (59 FR 7629, February 16, Director, Office of Pesticide Programs. Annex V contains provisions for the 1994). Therefore, 40 CFR chapter I is protection of specially designated areas Since tolerances and exemptions that amended as follows: specially managed areas and historic are established on the basis of a petition sites and monuments. Section 2405 of under FFDCA section 408(d), such as PART 180—[AMENDED] title 16 of the ACA directs the Director the tolerance exemption in this action, of the National Science Foundation to ■ do not require the issuance of a 1. The authority citation for part 180 issue such regulations as are necessary proposed rule, the requirements of the continues to read as follows: and appropriate to implement Annex V Regulatory Flexibility Act (5 U.S.C. 601 Authority: 21 U.S.C. 321(q), 346a and 371. to the Protocol. et seq.) do not apply. ■ 2. Add § 180.1365 to subpart D to read The Antarctic Treaty Parties, which This action directly regulates growers, as follows: includes the United States, periodically food processors, food handlers, and food adopt measures to establish, consolidate retailers, not States or tribes. -

Living and Working at USAP Facilities
Chapter 6: Living and Working at USAP Facilities CHAPTER 6: Living and Working at USAP Facilities McMurdo Station is the largest station in Antarctica and the southermost point to which a ship can sail. This photo faces south, with sea ice in front of the station, Observation Hill to the left (with White Island behind it), Minna Bluff and Black Island in the distance to the right, and the McMurdo Ice Shelf in between. Photo by Elaine Hood. USAP participants are required to put safety and environmental protection first while living and working in Antarctica. Extra individual responsibility for personal behavior is also expected. This chapter contains general information that applies to all Antarctic locations, as well as information specific to each station and research vessel. WORK REQUIREMENT At Antarctic stations and field camps, the work week is 54 hours (nine hours per day, Monday through Saturday). Aboard the research vessels, the work week is 84 hours (12 hours per day, Monday through Sunday). At times, everyone may be expected to work more hours, assist others in the performance of their duties, and/or assume community-related job responsibilities, such as washing dishes or cleaning the bathrooms. Due to the challenges of working in Antarctica, no guarantee can be made regarding the duties, location, or duration of work. The objective is to support science, maintain the station, and ensure the well-being of all station personnel. SAFETY The USAP is committed to safe work practices and safe work environments. There is no operation, activity, or research worth the loss of life or limb, no matter how important the future discovery may be, and all proactive safety measures shall be taken to ensure the protection of participants. -

Amundsen's 'South Pole': a Review Author(S): Hugh Robert Mill Review By: Hugh Robert Mill Source: the Geographical Journal, Vol
Review: Amundsen's 'South Pole': A Review Author(s): Hugh Robert Mill Review by: Hugh Robert Mill Source: The Geographical Journal, Vol. 41, No. 2 (Feb., 1913), pp. 148-151 Published by: geographicalj Stable URL: http://www.jstor.org/stable/1778869 Accessed: 22-05-2016 02:01 UTC Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Wiley, The Royal Geographical Society (with the Institute of British Geographers) are collaborating with JSTOR to digitize, preserve and extend access to The Geographical Journal This content downloaded from 132.77.150.148 on Sun, 22 May 2016 02:01:13 UTC All use subject to http://about.jstor.org/terms 148 AMUXDSEN'S 'SOUTH POLE ': A REVIEW. of Qatar. Wadi Duwasir and Wadi Nejran, called in its lower course Wadi Aftanah, both disappear in Robu el Khali, and Jebel Tuwariq runs all the way to Bisha and the bend of Wadi Duwasir. The full report of his journey will be awaited with interest. AMUNDSEN'S <SOUTH POLE '; A REVIEW. By HUGH ROBERT MILL, D.Sc. The two beautiful volumes in which Captain Amundsen and his comrades tell the story of the Norwegian Antarctic Expedition of 1910-12 * have appeared in English garb with an almost incredible promptitude. -

PECS Definitions and Rulings
POLAR EXPEDITIONS CLASSIFICATION SCHEME (PECS) ! DEFINITIONS AND RULINGS The Polar Expeditions Classification Scheme is a grading system for extended, unmotorised polar expeditions, crossings or circumnavigations, collectively referred to as Journeys. Polar regions, modes of travel, start and end points, routes and types of support are defined under the scheme and give expeditioners guidance on how to classify, promote and immortalise their journey. PECS uses three tiers of Designation to grade, label and describe polar journeys - a Label (made up of Label Elements), a Description and a MAP Code. Tiers are only an indication of information density. PECS does not discriminate between Modes of Travel. Each Mode is classified under the scheme allowing same-mode journeys to be compared while allowing for superficial cross-comparison. PECS is able to accommodate new modes of unmotorised travel as they develop without impacting on labelling or definitions. Journeys using engines or motors for propulsion, for any part of the journey, are not covered by PECS. PECS concentrates primarily on journeys of more than 400km in Antarctica, Greenland and on the Arctic Ocean however journeys in other polar areas and of less than 400km one-way linear distance that do not include the Poles or significant features on their line of travel may be classified on an informal basis under this scheme. Journeys choosing to use PECS must abide by PECS terminology. Shorter journeys should be labelled accordingly ie. Last Degree South Pole or Double Degree North Pole etc. All rulings and determinations are at the discretion of the PECS Committee. POLAR EXPEDITIONS CLASSIFICATION SCHEME "1 VER190220 CONTENTS 4. -
Public Information Leaflet HISTORY.Indd
British Antarctic Survey History The United Kingdom has a long and distinguished record of scientific exploration in Antarctica. Before the creation of the British Antarctic Survey (BAS), there were many surveying and scientific expeditions that laid the foundations for modern polar science. These ranged from Captain Cook’s naval voyages of the 18th century, to the famous expeditions led by Scott and Shackleton, to a secret wartime operation to secure British interests in Antarctica. Today, BAS is a world leader in polar science, maintaining the UK’s long history of Antarctic discovery and scientific endeavour. The early years Britain’s interests in Antarctica started with the first circumnavigation of the Antarctic continent by Captain James Cook during his voyage of 1772-75. Cook sailed his two ships, HMS Resolution and HMS Adventure, into the pack ice reaching as far as 71°10' south and crossing the Antarctic Circle for the first time. He discovered South Georgia and the South Sandwich Islands although he did not set eyes on the Antarctic continent itself. His reports of fur seals led many sealers from Britain and the United States to head to the Antarctic to begin a long and unsustainable exploitation of the Southern Ocean. Image: Unloading cargo for the construction of ‘Base A’ on Goudier Island, Antarctic Peninsula (1944). During the late 18th and early 19th centuries, interest in Antarctica was largely focused on the exploitation of its surrounding waters by sealers and whalers. The discovery of the South Shetland Islands is attributed to Captain William Smith who was blown off course when sailing around Cape Horn in 1819. -

The Polar Regions
TEACHING DOSSIER 1 ENGLISH, GEOGRAPHY, SCIENCE, ECONOMICS THE POLAR REGIONS ANTARCTIC, ARCTIC, GEOGRAPHY, CLIMATE, FAUNA, FLORA, CLIMATE CHANGE, THREATS, CONSERVATION NORTH POLE SOUTH POLE 2 dossier CZE N° 1 THEORY SECTION THE ARCTIC AND ANTARCTIC The Arctic and the Antarctic have a number of points in common: low temperatures, darkness that lasts for several weeks or months in winter, and magnificent expanses of ice... There are several different types of ice1, including sea ice, which is ice that contains salt, and ice caps and icebergs, which consist solely of freshwater ice. How- ever, once we get past these initial similarities, it doesn’t take long to realise that the Arctic and the Antarctic are two totally different regions. THE ARCTIC - Frozen ocean surrounded by land - North Pole: located approximately in the centre of the Arctic Ocean - Ocean covered to a large extent by permanent sea ice - Holds almost 10% of all the Earth’s continental ice (7% of the world’s reserves of freshwater) - Outer limit: place where the temperature never exceeds 10°C during the warmest month (July) - Area: 21 million km2 (14 million km2 of which is the Arctic Ocean) Ice drift Maximum extent of the sea ice in summer Maximum extent of the sea ice in winter Outer limit of the Arctic 10°C Figure 1: Outer limit of the Arctic and seasonal variation of the sea ice The Arctic Ocean is bordered by broad, shallow continental plates and consists of two main basins (4 km deep on average) separated by a range of underwater mountains: the Lomonosov Ridge, which joins the north of Greenland to the New Siberia Archipelago along a line that runs close to the North Pole. -

Antarctica: at the Heart of It All
4/8/2021 Antarctica: At the heart of it all Dr. Dan Morgan Associate Dean – College of Arts & Science Principal Senior Lecturer – Earth & Environmental Sciences Vanderbilt University Osher Lifelong Learning Institute Spring 2021 Webcams for Antarctic Stations III: “Golden Age” of Antarctic Exploration • State of the world • 1910s • 1900s • Shackleton (Nimrod) • Drygalski • Scott (Terra Nova) • Nordenskjold • Amundsen (Fram) • Bruce • Mawson • Charcot • Shackleton (Endurance) • Scott (Discovery) • Shackleton (Quest) 1 4/8/2021 Scurvy • Vitamin C deficiency • Ascorbic Acid • Makes collagen in body • Limits ability to absorb iron in blood • Low hemoglobin • Oxygen deficiency • Some animals can make own ascorbic acid, not higher primates International scientific efforts • International Polar Years • 1882-83 • 1932-33 • 1955-57 • 2007-09 2 4/8/2021 Erich von Drygalski (1865 – 1949) • Geographer and geophysicist • Led expeditions to Greenland 1891 and 1893 German National Antarctic Expedition (1901-04) • Gauss • Explore east Antarctica • Trapped in ice March 1902 – February 1903 • Hydrogen balloon flight • First evidence of larger glaciers • First ice dives to fix boat 3 4/8/2021 Dr. Nils Otto Gustaf Nordenskjold (1869 – 1928) • Geologist, geographer, professor • Patagonia, Alaska expeditions • Antarctic boat Swedish Antarctic Expedition: 1901-04 • Nordenskjold and 5 others to winter on Snow Hill Island, 1902 • Weather and magnetic observations • Antarctic goes north, maps, to return in summer (Dec. 1902 – Feb. 1903) 4 4/8/2021 Attempts to make it to Snow Hill Island: 1 • November and December, 1902 too much ice • December 1902: Three meant put ashore at hope bay, try to sledge across ice • Can’t make it, spend winter in rock hut 5 4/8/2021 Attempts to make it to Snow Hill Island: 2 • Antarctic stuck in ice, January 1903 • Crushed and sinks, Feb. -

Arcticorantarctic Lesson Plan
#ArcticorAntarctic Lesson Plan Created by Keechy Akkerman and Jean Polfus Can be adjusted depending on the age of the students. Time: up to 1 hour Materials - Blackboard & chalk or whiteboard or digital whiteboard or a globe or world map - Paper and colouring materials for the students - Potentially penguin and polar bear icons/markers if turned into a competition Plan - Start by asking the students if they know the name for the northern regions of the world or the southern regions of the world o Draw a very simple Arctic and Antarctic on the blackboard/whiteboard and ask the students to do the same on their paper and circle the Arctic and Antarctic o Use a globe or world map to point out the polar regions o - Ask the students what they know about the poles: things like cold, polar bears, penguins etc. - Next, the teacher can suggests certain elements that only belong to the Arctic, the Antarctic or both. The students can guess which one belongs where. The teacher can list these on the blackboard/whiteboard under columns for Arctic, Antarctic or Both (see suggestions below) or place the words or drawings on the map or blackboard/whiteboard diagram. The students can add drawings or words to their maps. - This could be turned into a competition where the classroom is split up in groups (for example the polar bears vs the penguins) and they battle for who gets the most answers correct. Suggestions for the game (more can be found on internet and difficulty can be adjusted depending on the age of the students). -

Nsf.Gov OPP-ANT: the U.S. Antarctic Program Today -- Logistics And
U.S. Antarctic Program Blue Ribbon Panel Review Overview The U.S. Antarctic Program (USAP), which is managed by the National Science Foundation (NSF), executes and provides oversight for numerous Antarctic science programs and the logistical and administrative resources to carry out the science mission. It also supports the U.S. policy of maintaining a permanent, peaceful presence in Antarctica. Current scientific efforts in Antarctica encompass a wide variety of fields and topics, from penguin population dynamics to astrophysics. The remote location and extreme environment require massive logistics support to every scientific endeavor. The USAP provides an umbrella capability that allows numerous government agencies to carry out scientific work in Antarctica and draws on the military and commercial sectors and cooperates with international partners to provide logistical and administrative support. The Office of Science and Technology Policy and the National Science Foundation are initiating a major review of the program to ensure that it continues to support the most relevant and important science in the most effective, efficient, sustainable, technologically advanced, innovative, safe, and environmentally-friendly manner, and to set the stage for the next two decades of U.S. research, discovery, and environmental stewardship in Antarctica. The results of the study will inform policy-makers and budget requests in FY 2013 and beyond. Called for in the FY 2010 President’s budget, this review will be conducted in two complementary phases. The first will be an examination of the scientific drivers that will shape the USAP science program in the coming decades and will be conducted by a panel formed by the National Research Council (NRC). -

Record Low Surface Air Temperature at Vostok Station, Antarctica
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 114, D24102, doi:10.1029/2009JD012104, 2009 Record low surface air temperature at Vostok station, Antarctica John Turner,1 Phil Anderson,1 Tom Lachlan-Cope,1 Steve Colwell,1 Tony Phillips,1 Ame´lie Kirchgaessner,1 Gareth J. Marshall,1 John C. King,1 Tom Bracegirdle,1 David G. Vaughan,2 Victor Lagun,3 and Andrew Orr1 Received 20 March 2009; revised 16 July 2009; accepted 11 September 2009; published 16 December 2009. [1] The lowest recorded air temperature at the surface of the Earth was a measurement of À89.2°C made at Vostok station, Antarctica, at 0245 UT on 21 July 1983. Here we present the first detailed analysis of this event using meteorological reanalysis fields, in situ observations and satellite imagery. Surface temperatures at Vostok station in winter are highly variable on daily to interannual timescales as a result of the great sensitivity to intrusions of maritime air masses as Rossby wave activity changes around the continent. The record low temperature was measured following a near-linear cooling of over 30 K over a 10 day period from close to mean July temperatures. The event occurred because of five specific conditions that arose: (1) the temperature at the core of the midtropospheric vortex was at a near-record low value; (2) the center of the vortex moved close to the station; (3) an almost circular flow regime persisted around the station for a week resulting in very little warm air advection from lower latitudes; (4) surface wind speeds were low for the location; and (5) no cloud or diamond dust was reported above the station for a week, promoting the loss of heat to space via the emission of longwave radiation.