Characteristics of Microbial Communities of Pachygrontha Antennata (Hemiptera: Pachygronthidae) in Relation to Habitat Variables
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Influence of Prairie Restoration on Hemiptera
CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Stephanie Kay Gunter, B.A. Dayton, Ohio August 2021 CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay APPROVED BY: Chelse M. Prather, Ph.D. Faculty Advisor Associate Professor Department of Biology Ryan W. McEwan, Ph.D. Committee Member Associate Professor Department of Biology Mark G. Nielsen Ph.D. Committee Member Associate Professor Department of Biology ii © Copyright by Stephanie Kay Gunter All rights reserved 2021 iii ABSTRACT CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay University of Dayton Advisor: Dr. Chelse M. Prather Ohio historically hosted a patchwork of tallgrass prairies, which provided habitat for native species and prevented erosion. As these vulnerable habitats have declined in the last 200 years due to increased human land use, restorations of these ecosystems have increased, and it is important to evaluate their success. The Hemiptera (true bugs) are an abundant and varied order of insects including leafhoppers, aphids, cicadas, stink bugs, and more. They play important roles in grassland ecosystems, feeding on plant sap and providing prey to predators. Hemipteran abundance and composition can respond to grassland restorations, age of restoration, and size and isolation of habitat. -

Changes in Arthropod Abundance and Diversity with Invasive
CHANGES IN ARTHROPOD ABUNDANCE AND DIVERSITY WITH INVASIVE GRASSES A Thesis by ERIN E. CORD Submitted to the College of Graduate Studies Texas A&M University-Kingsville in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2011 Major Subject: Range and Wildlife Management CHANGES IN ARTHROPOD ABUNDANCE AND DIVERSITY WITH INVASIVE GRASSES A Thesis by ERIN E. CORD Approved as to style and content by: ______________________________ Andrea R. Litt, Ph.D. (Chairman of Committee) ___________________________ ___________________________ Timothy E. Fulbright, Ph.D. Greta L. Schuster, Ph.D. (Member) (Member) _____________________________ Scott E. Henke, Ph.D. (Chair of Department) _________________________________ Ambrose Anoruo, Ph.D. (Associate VP for Research & Dean, College of Graduate Studies) August 2011 ABSTRACT Changes in Arthropod Abundance and Diversity with Invasive Grasses (August 2011) Erin E. Cord, B.S., University Of Delaware Chairman of Committee: Dr. Andrea R. Litt Invasive grasses can alter plant communities and can potentially affect arthropods due to specialized relationships with certain plants as food resources and reproduction sites. Kleberg bluestem (Dichanthium annulatum) is a non-native grass and tanglehead (Heteropogon contortus) is native to the United States, but recently has become dominant in south Texas. I sought to: 1) quantify changes in plant and arthropod communities in invasive grasses compared to native grasses, and 2) determine if grass origin would alter effects. I sampled vegetation and arthropods on 90 grass patches in July and September 2009 and 2010 on the King Ranch in southern Texas. Arthropod communities in invasive grasses were less diverse and abundant, compared to native grasses; I also documented differences in presence and abundance of certain orders and families. -

And Lepidoptera Associated with Fraxinus Pennsylvanica Marshall (Oleaceae) in the Red River Valley of Eastern North Dakota
A FAUNAL SURVEY OF COLEOPTERA, HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH FRAXINUS PENNSYLVANICA MARSHALL (OLEACEAE) IN THE RED RIVER VALLEY OF EASTERN NORTH DAKOTA A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By James Samuel Walker In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Entomology March 2014 Fargo, North Dakota North Dakota State University Graduate School North DakotaTitle State University North DaGkroadtaua Stet Sacteho Uolniversity A FAUNAL SURVEYG rOFad COLEOPTERA,uate School HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH Title A FFRAXINUSAUNAL S UPENNSYLVANICARVEY OF COLEO MARSHALLPTERTAitl,e HEM (OLEACEAE)IPTERA (HET INER THEOPTE REDRA), AND LAE FPAIDUONPATLE RSUAR AVSESYO COIFA CTOEDLE WOIPTTHE RFRAA, XHIENMUISP PTENRNAS (YHLEVTAENRICOAP TMEARRAS),H AANLDL RIVER VALLEY OF EASTERN NORTH DAKOTA L(EOPLIDEAOCPTEEAREA) I ANS TSHOEC RIAETDE RDI VWEITRH V FARLALXEIYN UOSF P EEANSNTSEYRLNV ANNOICRAT HM DAARKSHOATALL (OLEACEAE) IN THE RED RIVER VAL LEY OF EASTERN NORTH DAKOTA ByB y By JAMESJAME SSAMUEL SAMUE LWALKER WALKER JAMES SAMUEL WALKER TheThe Su pSupervisoryervisory C oCommitteemmittee c ecertifiesrtifies t hthatat t hthisis ddisquisition isquisition complies complie swith wit hNorth Nor tDakotah Dako ta State State University’s regulations and meets the accepted standards for the degree of The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of University’s regulations and meetMASTERs the acce pOFted SCIENCE standards for the degree of MASTER OF SCIENCE MASTER OF SCIENCE SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: David A. Rider DCoa-CCo-Chairvhiadi rA. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

LYGAEOIDEA La Superfamila Lygaeoidea (Hemiptera: Heteroptera: Pentatomomorpha) Es Una De Las Mayores Y Más Diver- Sas, Con Más De 4000 Especies, De Heteroptera
| 421 Resumen LYGAEOIDEA La superfamila Lygaeoidea (Hemiptera: Heteroptera: Pentatomomorpha) es una de las mayores y más diver- sas, con más de 4000 especies, de Heteroptera. Los hábitats de las especies del grupo son variados, hay grupos arbóreos, geófilos y laminófilos. La mayoría se alimentan de semillas maduras, aunque las Blissidae y algunas Lygaeidae son succionadoras de savia, los Geocoridae son principalmente depredadoras y las Cle- radini (Rhyparochromidae) se alimentan de sangre de vertebrados. Las ninfas viven en los mismos hábitats que los adultos y se alimentan generalmente de las mismas plantas. Actualmente en los Lygaeoidea se reconocen 15 familias, de las cuales 12 han sido registradas de la región Neotropical y 11 de la Argentina: Berytidae, Blissidae, Colobathristidae, Cymidae, Geocoridae, Lygaeidae, Ninidae, Oxycarenidae, Pachygronthidae, Piesmatidae y Rhyparochromidae. Se presenta una breve historia taxonómica, aspectos filogenéticos y de la clasificación actual de la superfamilia, bibliografía de referencia y una clave para la identificación de las familias de la Argentina. Para cada familia se presenta una diagnosis, principales trabajos, aspectos de la bio- logía y la diversidad a nivel mundial y en la Argentina, así como claves para la determinación de los géneros presentes en el pais. Además, se reseña la importancia agroeconómica del grupo. Se adjunta un listado de las 154 especies citadas de Argentina. Pablo Matías DELLAPÉ Abstract The superfamily Lygaeoidea (Hemiptera: Heteroptera: División Entomología, Museo de La Plata, Paseo del Pentatomomorpha) is one of the most diverse within the Bosque, 1900 La Plata, Argentina. Heteroptera, with more than 4000 species described. [email protected] The Lygaeoid habitats are diverse; there are arboreal, geophile and laminophile species. -

Species Distribution Ranking for the Multiple Projects Project Organism Group: Hemiptera Specimens
Species Distribution Ranking for the Multiple Projects Project Organism Group: Hemiptera Specimens Family and Species Sum Of Sites Where Species Was Found Cercopidae Clastoptera obtusa (Spittlebug) 26 Cercopidae Prosapia bicincta (Two-lined spittle bug) 24 Delphacidae Liburniella ornata (Planthopper) 21 Cicadellidae Jikradia olitorius (Leafhopper) 18 Miridae Lygus lineolaris (Tarnished plant bug) 18 Cercopidae Philaenus spumarius (Meadow spittlebug) 18 Berytidae Jalysus spinosus (Stilt bug) 18 Cercopidae Clastoptera xanthocephala (Spittlebug) 16 Cicadellidae Graphocephala coccinea (Leafhopper) 15 Pentatomidae Mormidea lugens (Stink bug) 12 Alydidae Alydus eurinus (Broad-headed bug) 12 Cercopidae Lepyronia quadrangularis (Spittlebug) 11 Pentatomidae Euschistus tristigmus (Stink bug) 11 Rhyparochromidae Pseudopachybrachius basalis (Seed bug) 10 Lygaeidae Kleidocerys resedae (Seed bug) 10 Psyllidae Cacopsylla carpinicola (Psyllid plant bug) 9 Rhopalidae Niesthrea louisianica (Scentless plant bug) 9 Cydnidae Sehirus cinctus (Burrower bug) 9 Cercopidae Aphrophora saratogenesis (Spittlebug) 9 Flatidae Metcalfa pruinosa (Planthopper) 9 Flatidae Anormenis chloris (Planthopper) 9 Psyllidae Bactericera tripunctata (Jumping plant lice) 8 Delphacidae Isodelphax basivitta (Planthopper) 8 Delphacidae Delphacodes puella (Planthopper) 8 Psyllidae Bactericera species (Jumping plant lice) 8 Cercopidae Aphrophora quadrinotata (Spittlebug) 8 Cercopidae Aphrophora cribrata (Pine spittle bug) 7 Pentatomidae Euschistus servus (Stink bug) 7 Membracidae Acutalis -
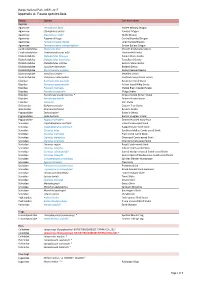
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

SPECIES LIST and STATE RECORDS of INSECTS COLLECTED from HOBCAW BARONY, SOUTH CAROLINA** University of Guelph Field Entomology Class, Spring of 2004
SPECIES LIST AND STATE RECORDS OF INSECTS COLLECTED FROM HOBCAW BARONY, SOUTH CAROLINA** University of Guelph Field Entomology Class, Spring of 2004. Gard Otis, Instructor; Steve Paiero, Assistant Instructor ORDER FAMILY Genus/Species Coleoptera Buprestidae Acmaeodera Coleoptera Buprestidae Brachys Coleoptera Buprestidae Buprestis salisburyensis Herbst, 1801 Coleoptera Buprestidae Haplanthaxia Coleoptera Buprestidae Haplanthaxia quercata (Fabricius) Coleoptera Buprestidae Taphrocerus Coleoptera Carabidae Calybe sallei (Chev.) Coleoptera Carabidae Panagaeus crucigerus Say Coleoptera Elateridae Alaus myops (Fabricius) Coleoptera Elateridae Cardiophorus Coleoptera Elateridae Melanotus Coleoptera Eucnemidae Dirrhagofarsus lewisi Reitter Coleoptera Monommidae Hyporhagus Coleoptera Oedemeridae Xanthochroa erythrocephala (Germar) Coleoptera Scarabaeidae Canthon laevis (Drury) Coleoptera Scarabaeidae Melanocanthon bispinatus (Robinson) Coleoptera Trogossitidae Tenebroides bimaculata Melsheimer Dermaptera Labiidae Labia Dermaptera Labiidae Labia cf. curvicauda (Motschulsky) Dermaptera Labiidae Vostox brunneipennis (Audinet-Serville, 1839) Diptera Asilidae Laphria saffrana Diptera Chloropidae Ectecephala Diptera Lauxaniidae Trigonometopus Diptera Micropezidae Grallipeza nebulosa (Loew) Diptera Micropezidae Taeniaptera Diptera Otitidae Chaetopsis Diptera Otitidae Delphinia picta (Fabricius) Diptera Otitidae Euxesta Diptera Otitidae Zacompsia fulva Coquillett Diptera Psilidae Loxocera cylindrica Say, 1823 Diptera Pyrgotidae Boreothrinax Diptera Syrphidae -

1 the RESTRUCTURING of ARTHROPOD TROPHIC RELATIONSHIPS in RESPONSE to PLANT INVASION by Adam B. Mitchell a Dissertation Submitt
THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell 1 A dissertation submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Entomology and Wildlife Ecology Winter 2019 © Adam B. Mitchell All Rights Reserved THE RESTRUCTURING OF ARTHROPOD TROPHIC RELATIONSHIPS IN RESPONSE TO PLANT INVASION by Adam B. Mitchell Approved: ______________________________________________________ Jacob L. Bowman, Ph.D. Chair of the Department of Entomology and Wildlife Ecology Approved: ______________________________________________________ Mark W. Rieger, Ph.D. Dean of the College of Agriculture and Natural Resources Approved: ______________________________________________________ Douglas J. Doren, Ph.D. Interim Vice Provost for Graduate and Professional Education I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Douglas W. Tallamy, Ph.D. Professor in charge of dissertation I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Charles R. Bartlett, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. Signed: ______________________________________________________ Jeffery J. Buler, Ph.D. Member of dissertation committee I certify that I have read this dissertation and that in my opinion it meets the academic and professional standard required by the University as a dissertation for the degree of Doctor of Philosophy. -

Biodiversity Summary: Burnett Mary, Queensland
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Ten Years of Heteroptera Barcoding at CBGP: Outputs and Prospects
Ten years of Heteroptera barcoding at CBGP: outputs and prospects. Jean-Claude Streito, Guénaëlle Genson, Eric Pierre, Astrid Cruaud, Jean Yves Rasplus To cite this version: Jean-Claude Streito, Guénaëlle Genson, Eric Pierre, Astrid Cruaud, Jean Yves Rasplus. Ten years of Heteroptera barcoding at CBGP: outputs and prospects.. 6. Quadrennial IHS Meeting, Dec 2018, La Plata, Argentina. hal-02788356 HAL Id: hal-02788356 https://hal.inrae.fr/hal-02788356 Submitted on 5 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ten years of Heteroptera barcoding at CBGP : outputs and prospects Streito Jean-Claude; Genson Guénaëlle; Pierre Éric; Cruaud Astrid & Rasplus Jean-Yves [email protected] : INRA-CBGP Montpellier IHS VI meeting La Plata 3-7 dec 2018 Barcoding at CBGP : 10 years My lab : INRA-CBGP (Centre for Biology and Management of Populations) Where : Montpellier, France My job : Produce tools for identification of Arthropods Morphology and molecular (barcoding) .02 [email protected] : INRA-CBGP Montpellier IHS VI meeting La Plata 3-7 dec 2018 Barcoding at CBGP : 10 years We are interested in Arthropods of agronomic interest Pests: Beneficial : Invasive : .03 [email protected] : INRA-CBGP Montpellier IHS VI meeting La Plata 3-7 dec 2018 Barcoding at CBGP : 10 years We have increased true bugs barcoding in recent years Many true bug species have become major pests in Europe in recent years, Pests: especially on fruit and vegetable crops Lygus spp., Nezara viridula, Gonocerus acuteangulatus .. -

Northern Rivers, New South Wales
Biodiversity Summary for NRM Regions Guide to Users Background What is the summary for and where does it come from? This summary has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. It highlights important elements of the biodiversity of the region in two ways: • Listing species which may be significant for management because they are found only in the region, mainly in the region, or they have a conservation status such as endangered or vulnerable. • Comparing the region to other parts of Australia in terms of the composition and distribution of its species, to suggest components of its biodiversity which may be nationally significant. The summary was produced using the Australian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. The list of families covered in ANHAT is shown in Appendix 1. Groups notnot yet yet covered covered in inANHAT ANHAT are are not not included included in the in the summary. • The data used for this summary come from authoritative sources, but they are not perfect.