Human C1q A, B, and C Chains of Carboxyl-Terminal, Globular Head Region Modular Organization Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Friends, Enemies, and Fools: a Collection of Uyghur Proverbs by Michael Fiddler, Zhejiang Normal University
GIALens 2017 Volume 11, No. 3 Friends, enemies, and fools: A collection of Uyghur proverbs by Michael Fiddler, Zhejiang Normal University Erning körki saqal, sözning körki maqal. A beard is the beauty of a man; proverbs are the beauty of speech. Abstract: This article presents two groups of Uyghur proverbs on the topics of friendship and wisdom, to my knowledge only the second set of Uyghur proverbs published with English translation (after Mahmut & Smith-Finley 2016). It begins with a brief introduction of Uyghur language and culture, then a description of Uyghur proverb styles, then the two sets of proverbs, and finally a few concluding comments. Key words: Proverb, Uyghur, folly, wisdom, friend, enemy 1. Introduction Uyghur is spoken by about 10 million people, mostly in northwest China’s Xinjiang Uyghur Autonomous Region. It is a Turkic language, most closely related to Uzbek and Salar, but also sharing similarity with its neighbors Kazakh and Kyrgyz. The lexicon is composed of about 50% old Turkic roots, 40% relatively old borrowings from Arabic and Persian, and 6% from Russian and 2% from Mandarin (Nadzhip 1971; since then, the Russian and Mandarin borrowings have presumably seen a slight increase due to increased language contact, especially with Mandarin). The grammar is generally head-final, with default SOV clause structure, A-N noun phrases, and postpositions. Stress is typically word-final. Its phoneme inventory includes eight vowels and 24 consonants. The phonology includes vowel harmony in both roots and suffixes, which is sensitive to rounded/unrounded (labial harmony) and front/back (palatal harmony), consonant harmony across suffixes, which is sensitive to voiced/unvoiced and front/back distinctions; and frequent devoicing of high vowels (see Comrie 1997 and Hahn 1991 for details). -

A Review of Heat Stroke and Its Complications in the Canine Renee L
Volume 45 | Issue 1 Article 1 1983 A Review of Heat Stroke and Its Complications in the Canine Renee L. Larson Iowa State University Robert W. Carithers Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/iowastate_veterinarian Part of the Small or Companion Animal Medicine Commons, and the Veterinary Physiology Commons Recommended Citation Larson, Renee L. and Carithers, Robert W. (1983) "A Review of Heat Stroke and Its Complications in the Canine," Iowa State University Veterinarian: Vol. 45 : Iss. 1 , Article 1. Available at: https://lib.dr.iastate.edu/iowastate_veterinarian/vol45/iss1/1 This Article is brought to you for free and open access by the Journals at Iowa State University Digital Repository. It has been accepted for inclusion in Iowa State University Veterinarian by an authorized editor of Iowa State University Digital Repository. For more information, please contact [email protected]. A Review of Heat Stroke and Its Complications in the Canine Renee L. Larson, BS * Robert W. Carithers, DVM, MS, PhD** SUMMARY most obvious prerequisite is a high en A review of heat stroke and its complications vironmental temperature. When the ambient is presented. The etiology, physiology, clinical temperature increases above 86°F, a rise in in signs, secondary complications, diagnosis, ternal body temperature results. Dogs can treatment, necropsy results and prevention of tolerate rising environmental temperature heat stroke are discussed. A clinical case is quite well. However, when the body tempera then presented to illustrate the disorder of heat ture exceeds 104°F a breakdown of the stroke. animal's thermal equilibrium begins. -

T'ahsíi Gha Gots'ęh Náets'ehndı́h Gha Goɂǫ
CANADA GOGHA AMÍI GOHÉH DZÁA NĮONIDHE SÍI MEGHA ?E?Á T’AHSÍI GHA GOTS’ĘH NÁETS’EHNDÍH GHA GOɁǪ agots’ı̨ la t’áh ǫkı edáidzę gots’ę́ gots’áendíh íle gha T’ahsíi gha gots’ęh naets’éhndı́h énidé gots’áendıh́ ts’ęh edı̨ htł’éh gogháts’ı̨ ɂa gots’ęh gha go ǫ azhíi ǫt’e? eghálats’enda íle gha godı̨ eghálats’enda éhsíi edı̨ htł’éh ɂ seets’eleh gogháts’ıdhah.́ T’ahsíi goghǫ ts’enéhe T’ahsíi gha gots’ęh náets’éhndıh́ gha goɂǫ énidé t’áh dádéhtí íle énidé gots’ęh t’ahsíi tsıts’ı́ ̨ hthe dádéhtí amíi méh dzaah nıgot’ǫ́ ts’ıhɂǫh gosaamba hule agújá, náts’ehndíh mets’ęh edı̨ htł’éh ats’ehɂı̨ gogháts’ı̨ ɂá íle amíi mets’ę́ t’áhsíi ts’enıdhę t’áh. T’ahsíi gha gots’ęh énidé dádétí náíndí ts’enidhę t’áh gots’ęnoendíh. náets’éhndıh́ gha goɂǫ énidé t’áh amíi t’ahsíi tsı̨ guıdhe meghǫh názhaetı gots’eh zǫ́ dúle gots’ęh noendıh́ Gohseenízhaehti adi t’áh amíi mehéh dzaah nıgot’ǫ́ t’áh ats’eleh. Amíi t’ahsíi tsı̨ gųihthe meghǫnázhaeti k’eodı̨ ɂá saamba ts’ęhke mesaamba hule énidé dúle edi káondıh́ ǫt’e gots’ęh dúle thahne t’áh meghǫnázhaetı t’áh t’áh gots’áhodi: ats’eleh íle énidé t’ahsíi tśı̨ ts’ıhthé t’áh goghǫnázhaetı • Dzaah níots’éniɂǫ t’áh t’ahsí gots’ęh ts’enıdhę t’ah gǫndi k’ę́ ę́ ats’et’ı̨̨ gha góɂǫ íle énidé goghǫnázhaeti íle énidé godı̨ náts’edéh gokųę theɂǫ goch’á t’áh gogha gǫndi ts’ehtsi. -

Theories of Infant Development Theories of Infant Development
Theories of Infant Development Theories of Infant Development Edited by Gavin Bremner and Alan Slater © 2004 by Blackwell Publishing Ltd except for editorial material and organization © 2004 by Gavin Bremner and Alan Slater 350 Main Street, Malden, MA 02148-5020, USA 108 Cowley Road, Oxford OX4 1JF, UK 550 Swanston Street, Carlton, Victoria 3053, Australia The right of Gavin Bremner and Alan Slater to be identified as the Authors of the Editorial Material in this Work has been asserted in accordance with the UK Copyright, Designs, and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs, and Patents Act 1988, without the prior permission of the publisher. First published 2004 by Blackwell Publishing Ltd Library of Congress Cataloging-in-Publication Data Theories of infant development / edited by Gavin Bremner and Alan Slater. p. cm. Includes bibliographical references and index. ISBN 0–631–23337–7 (hc : alk. paper) — ISBN 0–631–23338–5 (pbk. : alk. paper) 1. Infants—Development. 2. Child development. I. Bremner, J. Gavin, 1949– II. Slater, Alan. RJ134.T48 2003 305.232—dc21 2003045328 A catalogue record for this title is available from the British Library. Set in 10/12 pt Palatino by Graphicraft Limited, Hong Kong Printed and bound in the United Kingdom by TJ International, Padstow, Cornwall. For further information on Blackwell Publishing, visit our website: http://www.blackwellpublishing.com In memory of George Esmond Butterworth, November 8, 1946–February 12, 2000 Contents Contributors ix Preface xi Part I Development of Perception and Action 1 A Dynamical Systems Perspective on Infant Action and its Development 3 Eugene C. -

Regulation GHA-RA, Maintenance of the Position Classification Plan
GHA-RA MONTGOMERY COUNTY REGULATION PUBLIC SCHOOLS Related Entries: Responsible Office: Human Resources and Development Maintenance of the Position Classification Plan I. PURPOSE To establish procedures for determining a position classification, reclassifying an existing position, reconstituting a position, classifying a proposed additional position, and writing and revising position specifications II. DEFINITIONS A. An appropriate officer is the superintendent of schools, deputy superintendent, chief operating officer, associate superintendent, department director, principal, or immediate supervisor. B. Classification/reclassification is a process to determine salary grades. A reclassification study involves identifying a significant change in the duties and responsibilities of a position that may require additional knowledge, skills, and abilities as a result of reorganization, new technologies, program revisions, and other events that impact the nature of the work to be performed that may or may not result in a salary grade change. C. Reconstitution involves assigning an existing or vacant position to a more appropriate classification and salary grade or the establishment of a new classification, typically as the result of reorganization or restructuring of an office or work unit. III. PROCEDURES A. Determination of a Position Classification Positions are included in the same classification when the same descriptive title applies, the same salary grade is equitable for all of the positions in the classification, and the following are substantially similar: 1 of 5 GHA-RA 1. Duties and responsibilities of the positions 2. Education and experience required 3. Tests or other methods used to determine applicants’ knowledge, abilities, and skills B. Reclassification of an Existing Position 1. Each December, the Office of Human Resources and Development (OHRD) with the approval of the chief operating officer sends a memorandum to the appropriate officers which outlines that year’s reclassification study process. -

GHA Fact Sheet Series Section 3 Program
For information: Justin Herter Public Information Officer (409) 765-1923 [email protected] GHA Fact Sheet Series Section 3 Program The GHA is currently assessing how to develop a Section 3 business and/or train and utilize Section 3 residents in its redevelopment initiatives. Since the storm, the GHA has developed a revitalization plan that includes the redevelopment of four public housing sites and up to 179 scattered site housing units throughout the island. These initiatives will be a combination of both new construction and rehabilitation of vacant structures in need of repair. By working to rebuild properties, the GHA will be in an incredible position to contribute to the economic recovery on the island as well as increase the affordable housing opportunities. Section Three of the Housing and Urban Development Act of 1968, as amended by the Housing & Community Development Act of 1994, requires that, to the greatest extent feasible, employment and other economic opportunities generated by HUD funds be directed to low and very low income residents. 24 CFR Part 135 requires that the GHA make best efforts to ensure that 30% of new hires of the GHA and of its contractors funded through development assistance, operating assistance or modernization assistance from HUD be residents of GHA communities. Furthermore, it requires that best efforts be made to ensure that 10% of all construction or repair related contracts and 3% of all other contracts be awarded to Section 3 business concerns. A Section Three Business Concern is defined as a business concern that is 51% owned by a GHA or other Section 3 residents, or 30% of whose permanent full time employees are GHA or other Section 3 residents or have been within the past three years. -

Georgia Coverdell Acute Stroke Registry Quarterly Newsletter WINTER 2013 Coverdell Partners: Georgia Coverdell 2013 Awards
Georgia Coverdell Acute Stroke Registry Quarterly Newsletter WINTER 2013 Coverdell Partners: Georgia Coverdell 2013 Awards Georgia Department of Public Health “Georgia Coverdell Champion Hospital of the Year” (DPH) Award Winners Emory University School of Medicine CONGRATULATIONS TO THE FOLLOWING 4 HOSPITALS: Georgia Medical Care Foundation Putnam General Hospital (very small hospital, 25 beds or less) (GMCF) American Stroke Dekalb Medical Hillandale (small hospital, 26–100 beds) Association (ASA) Georgia Hospital South Georgia Medical Center (medium hospital, 101–350 beds) Association (GHA) Grady Health System (large hospital, over 350 beds) If you have anything you would All Georgia Coverdell hospitals have the opportunity to receive the award, which is like included in an based on a point system. A total of four hospitals receiving the most points during upcoming the period from January 1, 2013 through December 31, 2013 will be the recipients newsletter or have of next year’s award. The award is a framed certificate to share with your hospital achieved recent and hospital administration. recognition in the area of stroke, The point system is based on the following criteria: contact: Participation Points Allotted Hospital attendance on GA Monthly Coverdell Call 1 Kerrie Krompf Hospital presenting on GA Monthly Coverdell Call 5 [email protected] Physician Champion presenting on GA Monthly Coverdell Call 10 Published Q-Tip in Coverdell Quarterly Newsletter 5 or Published Article in Coverdell Quarterly Newsletter 10 Published “Blurb” (250 words) in Coverdell Quarterly Newsletter 2 770-380-8998 Published Stroke Survivor Story in Coverdell Quarterly Newsletter 10 Workshop attendance (per hospital) 5 In the event that multiple articles, blurbs, stroke survivor stories, Q-tips, etc. -

GHA Letterhead Template
Depression Screening Background As many as 1 in 6 people over 65 suffer from depression. Estimates show depression occurs in 25% of older Americans with other illness, such as: • Cancer • Arthritis • Stroke • Chronic lung disease • Cardiovascular disease • Chronic pain Factors such as loss of loved ones or friends increase the likelihood of depression. Health care providers miss opportunities to improve mental health and general medical outcomes when mental illness is under-recognized and undertreated in primary care settings. Depression Screening Utilization Data analysis shows WPS GHA providers in Jurisdiction 5 (J5) and Jurisdiction 8 (J8) are underutilizing depression screening. The 2019 rate of depression screening ranges from 1.8% to 6.4% of beneficiaries. See below for specific state data. The average screening rate of all beneficiaries is: • Jurisdiction 5 (J5) is about 2.8% • Jurisdiction 8 (J8) is about 3.8% • National screening rate is 5.44% for all Medicare Administrative Contractors (MACs). Providers in J5 and J8 underutilize depression screening, while the prevalence of depression is relatively high. The range is from 17% in Nebraska to 20.4% in Indiana and Missouri. The rates of all states except for that of Nebraska, are higher than the national rate of 17.9%. Depression Screening Coverage Criteria The following coverage criteria apply: • Anyone eligible for Medicare • Screening only. It does not apply to: o Depression treatment o Chronic condition caused by depression • One year between screenings o 11 full months must pass following the month in which the screening occurred o Example: depression screening on 7/12/2019, next available 7/1/2020 • Furnished in a primary care setting with staff assisted depression care to ensure o Accurate diagnosis o Effective treatment o Follow-up • Up to 15-minute screening o This includes time spent to: . -
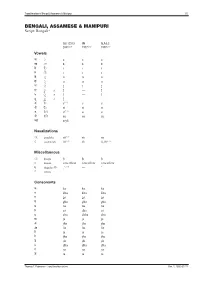
Transliteration of <Script Name>
Transliteration of Bengali, Assamese & Manipuri 1/5 BENGALI, ASSAMESE & MANIPURI Script: Bengali* ISO 15919 UN ALA-LC 2001(1.0) 1977(2.0) 1997(3.0) Vowels অ ◌ a a a আ ◌া ā ā ā ই ি◌ i i i ঈ ◌ী ī ī ī উ ◌ু u u u ঊ ◌ূ ū ū ū ঋ ◌ৃ r̥ ṛ r̥ ৠ ◌ৄ r̥̄ — r̥̄ ঌ ◌ৢ l̥ — l̥ ৡ ◌ৣ A l̥ ̄ — — এ ে◌ e(1.1) e e ঐ ৈ◌ ai ai ai ও ে◌া o(1.1) o o ঔ ে◌ৗ au au au অ�া a:yā — — Nasalizations ◌ং anunāsika ṁ(1.2) ṁ ṃ ◌ঁ candrabindu m̐ (1.2) m̐ n̐, m̐ (3.1) Miscellaneous ◌ঃ bisarga ḥ ḥ ḥ ◌্ hasanta vowelless vowelless vowelless (1.3) ঽ abagraha Ⓑ :’ — ’ ৺ isshara — — — Consonants ক ka ka ka খ kha kha kha গ ga ga ga ঘ gha gha gha ঙ ṅa ṅa ṅa চ ca cha ca ছ cha chha cha জ ja ja ja ঝ jha jha jha ঞ ña ña ña ট ṭa ṭa ṭa ঠ ṭha ṭha ṭha ড ḍa ḍa ḍa ঢ ḍha ḍha ḍha ণ ṇa ṇa ṇa ত ta ta ta Thomas T. Pedersen – transliteration.eki.ee Rev. 2, 2005-07-21 Transliteration of Bengali, Assamese & Manipuri 2/5 ISO 15919 UN ALA-LC 2001(1.0) 1977(2.0) 1997(3.0) থ tha tha tha দ da da da ধ dha dha dha ন na na na প pa pa pa ফ pha pha pha ব ba ba ba(3.2) ভ bha bha bha ম ma ma ma য ya ja̱ ya র ⒷⓂ ra ra ra ৰ Ⓐ ra ra ra ল la la la ৱ ⒶⓂ va va wa শ śa sha śa ষ ṣa ṣha sha স sa sa sa হ ha ha ha ড় ṛa ṙa ṛa ঢ় ṛha ṙha ṛha য় ẏa ya ẏa জ় za — — ব় wa — — ক় Ⓑ qa — — খ় Ⓑ k̲h̲a — — গ় Ⓑ ġa — — ফ় Ⓑ fa — — Adscript consonants ◌� ya-phala -ya(1.4) -ya ẏa ৎ khanda-ta -t -t ṯa ◌� repha r- r- r- ◌� baphala -b -b -b ◌� raphala -r -r -r Vowel ligatures (conjuncts)C � gu � ru � rū � Ⓐ ru � rū � śu � hr̥ � hu � tru � trū � ntu � lgu Thomas T. -

Balinese Romanization Table
Balinese Principal consonants1 (h)a2 ᬳ ᭄ᬳ na ᬦ ᭄ᬦ ca ᬘ ᭄ᬘ᭄ᬘ ra ᬭ ᭄ᬭ ka ᬓ ᭄ᬓ da ᬤ ᭄ᬤ ta ᬢ ᭄ᬢ sa ᬲ ᭄ᬲ wa ᬯ la ᬮ ᭄ᬙ pa ᬧ ᭄ᬧ ḍa ᬟ ᭄ᬠ dha ᬥ ᭄ᬟ ja ᬚ ᭄ᬚ ya ᬬ ᭄ᬬ ña ᬜ ᭄ᬜ ma ᬫ ᭄ᬫ ga ᬕ ᭄ᬕ ba ᬩ ᭄ᬩ ṭa ᬝ ᭄ᬝ nga ᬗ ᭄ᬗ Other consonant forms3 na (ṇa) ᬡ ᭄ᬡ ca (cha) ᭄ᬙ᭄ᬙ ta (tha) ᬣ ᭄ᬣ sa (śa) ᬱ ᭄ᬱ sa (ṣa) ᬰ ᭄ᬰ pa (pha) ᬨ ᭄ᬛ ga (gha) ᬖ ᭄ᬖ ba (bha) ᬪ ᭄ᬪ ‘a ᬗ᬴ ha ᬳ᬴ kha ᬓ᬴ fa ᬧ᬴ za ᬚ᬴ gha ᬕ᬴ Vowels and other agglutinating signs4 5 a ᬅ 6 ā ᬵ ᬆ e ᬾ ᬏ ai ᬿ ᬐ ĕ ᭂ ö ᭃ i ᬶ ᬇ ī ᬷ ᬈ o ᭀ ᬑ au ᭁ ᬒ u7 ᬸ ᬉ ū ᬹ ᬊ ya, ia8 ᭄ᬬ r9 ᬃ ra ᭄ᬭ rĕ ᬋ rö ᬌ ᬻ lĕ ᬍ ᬼ lö ᬎ ᬽ h ᬄ ng ᬂ ng ᬁ Numerals 1 2 3 4 5 ᭑ ᭒ ᭓ ᭔ ᭕ 6 7 8 9 0 ᭖ ᭗ ᭘ ᭙ ᭐ 1 Each consonant has two forms, the regular and the appended, shown on the left and right respectively in the romanization table. The vowel a is implicit after all consonants and consonant clusters and should be supplied in transliteration, unless: (a) another vowel is indicated by the appropriate sign; or (b) the absence of any vowel is indicated by the use of an adeg-adeg sign ( ). (Also known as the tengenen sign; ᭄ paten in Javanese.) 2 This character often serves as a neutral seat for a vowel, in which case the h is not transcribed. -

GHA-743 Power Riveter
GHA-743 Power Riveter OPERATING MAINTENANCE MANUAL 1224 East Warner Ave, Santa Ana, Ca 92707 Tel: 1-714-850-6022 [email protected] GHA-743 Power Riveter The Avdel Cherry GHA-743 is a pneumatic- hydraulic tool designed specifically for the most efficient installation of Avdel Cherry Commercial fasteners. It weighs only 61/4 pounds and can be operated in any position with one hand. It has a full 3/4" rivet setting stroke and a rated pull load of 3600 pounds. The GHA-743 riveter operates on a wide range of air pressure, with 90 to 120 psi providing the maximum efficiency. At 90 lbs. air pressure, the GHA-743 has a decibel rating of 82dB(A) and consumes 6 CFM at 20 cycles per minute. A mandrel catcher bag may be attached to eliminate costly cleanup. The GHA-743 comes equipped with nose- piece 743A7-8Z ready to set: Avdel Cherry N Rivets -1/4" diameter Avdel Cherry Q Rivets - 1/4" diameter Warning: Approved protection should be worn when operating, repairing, or overhauling this tool. There is also a spare nosepiece, 743A7- 5C6Z included for setting: Avdel Cherry N Rivets - 3/16" diameter Avdel Cherry Q Rivets - 3/16" diameter MANY OTHER STYLES AND SIZES OF AVDEL CHERRY COMMERCIAL RIVETS CAN BE SET WITH THIS TOOL. REFER TO THE NOSEPIECE SELECTION CHART ON PAGE10 FOR APPLICABLE PART NUMBERS. 1 How the GHA-743 Operates: When the tool is pressurized by hooking up to an air valve, it in turn activates the hydraulic power air line, the valve spool is forced upward. -

May 26, 2021 GHA Seed Fund List When Requesting Seeds from Mel
May 26, 2021 GHA seed fund list When requesting seeds from Mel Grice, if you do not give him your snail mail address, you will NOT receive any seeds. GHA is not certain of your address unless you tell Mel what it is each and every time you order seeds. Mel Grice <[email protected]> Because there is usually a big rush for ordering seeds when the CrossWords newsletter is sent, you must give at least 10 substitutions. Often Mel receives a couple dozen seeds from one cross, so supplies could be limited. Keep in mind that the request limit is five (5) packets each time CrossWords is mailed. Since GHA is absorbing the costs of mailing, please make no more than 3 requests a year with a maximum of 5 packets (but send 10 substitute requests per time). Clay Anderson, USA CA 14-01 Streptocarpus ‘Iced Artistry’ x ‘Renia’ CA 14-02 Streptocarpus ‘Iced Artistry’ x ‘Gaucho’ CA 14-05 Streptocarpus ‘Neil’s Hot Flash’ x ‘Renia’ CA 14-06 Streptocarpus ‘Neil’s Hot Flash’ x ‘Gaucho’ CA 20-02 Sinningia ([guttata x helleri] x helleri) x ([cardinalis ‘Skydiver’ x macrorrhiza*] x glazioviana) *note — S. macrorrhiza is a superseded synonym for S. bulbosa Nancy Block, USA NB 06-04 Streptocarpus ‘Canterbury Surprise’ x S. fasciatus NB 06-12 Streptocarpus ‘Great Expectations’ x unnamed double NB 07-05 Streptocarpus 'Iced Pink Flamingo' x S. 'Bethan' NB 08-05 Streptocarpus ST 04-07 x JEP 05-03 NB 11-03 Streptocarpus (Mixed seeds combined from 2010 crosses) Betty Cessna, USA BC 12-07 Streptocarpus — Mixed seeds Karyn Cichocki, USA KC 19-03 Sinningia ‘Towering Inferno’ x ? (open pollinated) KC 19-04 Sinningia sellovii hybrids mix (open pollinated) KC 19-06 Sinningia brasiliensis x S.