<I>ARGUSIANUS ARGUS</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Document Resume Ed 049 958 So 000 779 Institution Pub
DOCUMENT RESUME ED 049 958 SO 000 779 AUTHCE Nakosteen, Mehdi TITLE Conflicting Educational Ideals in America, 1775-1831: Documentary Source Book. INSTITUTION Colorado Univ., Boulder. School of Education. PUB DATE 71 NOTE 480p. EDES PRICE EDES Price MF-SC.65 HC-$16.45 DESCRIPTORS *Annotated Bibliographies, Cultural Factors, *Educational History, Educational Legislation, *Educational Practice, Educational Problems, *Educational Theories, Historical Reviews, Resource Materials, Social Factors, *United States History IDENTIFIERS * Documentary History ABSTRACT Educational thought among political, religious, educational, and other social leaders during the formative decades of American national life was the focus of the author's research. The initial objective was the discovery cf primary materials from the period to fill a gap in the history of American educational thought and practice. Extensive searching cf unpublished and uncatalogued library holdings, mainly those of major public and university libraries, yielded a significant quantity of primary documents for this bibliography. The historical and contemporary works, comprising approximately 4,500 primary and secondary educational resources with some surveying the cultural setting of educational thinking in this period, are organized around 26 topics and 109 subtopics with cross-references. Among the educational issues covered by the cited materials are: public vs. private; coed vs. separate; academic freedom, teacher education; teaching and learning theory; and, equality of educational opportunity. In addition to historical surveys and other secondary materials, primary documents include: government documents, books, journals, newspapers, and speeches. (Author/DJB) CO Lir\ 0 CY% -1- OCY% w CONFLICTING EDUCATIONAL I D E A L S I N A M E R I C A , 1 7 7 5 - 1 8 3 1 : DOCUMENTARY SOURCE B 0 0 K by MEHDI NAKOSTEEN Professor of History and Philosophy of Education University of Colorado U.S. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Argus 2019 Q1
The Argus January 2019 A.S. LIII _____________________________________________________ Barony of One Thousand Eyes, Kingdom of Artemisia “The Heart of Artemisia” This is theJanuary 2019 issue of the Argus, a publication of the Barony of one Thousand Eyes, Kingdom of Artemesia, Society for Creative Anachronism, Inc. and is available from Lisa Edmiston, [email protected]. It is not a corporate publication of the Society for Creative Anachronism, Inc. and does not delineate SCA, Inc. policies. Copyright 2019, Society for Creative Anachronism Inc. For information on reprinting photographs, articles, or artwork from this publication please contact the Chronicler, who will assist you in contacting the original creator of the piece. Please respect the legal rights of our contributors. Join The Current Middle Ages! The Society for Creative Anachronism, or SCA, is an international organization dedicated to researching and re-creating the arts, skills, and traditions of pre-17th-century Europe. Members of the SCA study and take part in a variety of activities, including combat, archery, equestrian activities, costuming, cooking, metalwork, woodworking, music, dance, calligraphy, fiber arts, and much more. If it was done in the Middle Ages or Renaissance, odds are you’ll find someone in the SCA interested in recreating it. You will frequently hear SCA participants describe the SCA as recreating the Middle Ages “as they ought to have been.” In some ways this is true – we choose to use indoor plumbing, heated halls, and sewing -

Testing Darwin's Hypothesis on the Evolution of Ornamental Eyespots In
Testing Darwin’s hypothesis on the evolution of ornamental eyespots in peafowl and their relatives Rebecca T. Kimball, Keping Sun, Kelly Meicklejohn, Edward Braun, Brant Faircloth, Travis Glenn Darwin • “As no ornaments are more beautiful than the ocelli on the feather of various birds…they deserve to be especially noticed.” (The Descent of Man: And Selection in Relation to Sex) Hypothesis • Darwin suggested a gradual evolution from the simple spots and ocelli of some species, to the magnificent ocelli of peafowl Ocelli in other taxa • Noted similarities between peafowl (Pavo), argus pheasant (Argusianus), and peacock pheasants (Polyplectron) Relationships • Based on the shared ocelli, some classifications have united the peafowl, peacock pheasants and argus pheasants into a clade • However, molecular phylogenies have not been able to resolve relationships of these taxa with confidence What we do know • Based on molecular phylogenies: – The peafowl are most closely related to the Congo peafowl (which lacks ocelli) – The argus pheasant is most closely related to the crested argus (which lacks ocelli) • So the simplest interpretation of Darwin’s ideas, that the ocellated taxa would be each other’s closest relatives, is not supported Ocelli can be lost • Within the peacock pheasants, one species lacks complex ocelli – This appears to be a derived species – So loss of ocelli does occur • A modification of Darwin’s hypothesis might be that ocelli evolved once, but have been lost in Congo peacock, crested argus, and one species of peacock pheasant -

Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa
Journal of Heredity 2003:94(6):472–489 Ó 2003 The American Genetic Association DOI: 10.1093/jhered/esg092 Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa K. L. BUSH AND C. STROBECK From the Department of Biological Sciences, University of Alberta–Edmonton, Edmonton, Alberta T6G 2E9, Canada. Address correspondence to Krista Bush at the address above, or e-mail: [email protected]. Abstract The phylogenetic relationships of 21 pheasant and 6 non-pheasant species were determined using nucleotide sequences from the mitochondrial cytochrome b gene. Maximum parsimony and maximum likelihood analysis were used to try to resolve the phylogenetic relationships within Phasianidae. Both the degree of resolution and strength of support are improved over previous studies due to the testing of a number of species from multiple pheasant genera, but several major ambiguities persist. Polyplectron bicalcaratum (grey peacock pheasant) is shown not to be a pheasant. Alternatively, it appears ancestral to either the partridges or peafowl. Pucrasia macrolopha macrolopha (koklass) and Gallus gallus (red jungle fowl) both emerge as non-pheasant genera. Monophyly of the pheasant group is challenged if Pucrasia macrolopha macrolopha and Gallus gallus are considered to be pheasants. The placement of Catreus wallichii (cheer) within the pheasants also remains undetermined, as does the cause for the great sequence divergence in Chrysolophus pictus obscurus (black-throated golden). These results suggest that alterations in taxonomic -
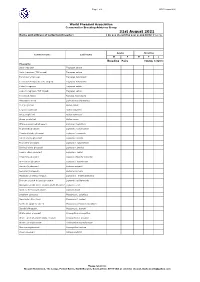
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

The Argus Pheasant Is at Times One of the Most Ornamental Member of the En Tire Pheasant Family
c ~ CJ) :> > () c ro Z > .D C/) o .!:: 0.. by Francis Billie The Argus Pheasant is at times one of the most ornamental member of the en tire Pheasant Family. Adult males can measure ix to even feet from the tip of the beak to the tip of the tail, although most of this length is taken up by their tail feather . In coloration the Argus is rather dull and plain, it is only during its marvelous courtship display that it manifest its great beauty and elaborate markings. There are two genera of Argus each containing two subspecies. In the genus Rehinartia there is Rheinart's Crested Close-up feather detads of the Arj?us Pheasant. Argus Pheasant, male photos by Francis Billie Argus Pheasant, hen 38 Argus, (Rheinartia ocellata ocellata), back and forth and he vibrates his found in Vietnam and parts of Laos, and plumage so the ocelli appear to revolve. the Malay Crested Argus (Rheinartia During this display the male's head is hid ocellalla nigrescens) a rare, little known den from view. bird found in various parts of the Malay It is interesting to note that the Argus Peninsula. At present, the status of the display is not like that of the Peacock bird in Vietnam is unknown, but the Pheasants in that the frontal display is not made with the tail feathers but is formed ,c F P r I Malayan subspecies has been observed in '{;HI \ t ') the wild state during a recent expedition in with the wings. It is also interesting that the f'PICrS & 81RUS the area. -

Illegal Wildlife Trade 02
2019 02 Illegal Wildlife Trade Wildlife Illegal NEWS 1 WAZA Executive Office Staff Interim CEO and Martín Zordan Director of Conservation [email protected] Chief Operating Officer Christina Morbin [email protected] Director of Communications Gavrielle Kirk-Cohen [email protected] Director of Membership Janet Ho [email protected] Animal Welfare and Paula Cerdán Conservation Coordinator [email protected] Imprint WAZA Executive Office Contacts Postal Address WAZA Executive Office Editor: Carrer de Roger de Llúria 2, 2-2 Gavrielle Kirk-Cohen 08010 Barcelona Spain Proofreader Phone +34 936638811 Laurie Clinton Email [email protected] Website www.waza.org Layout and design: Facebook www.facebook.com/officialWAZA Butterhalfsix.com Twitter twitter.com/WAZA This edition of WAZA News is Instagram www.instagram.com/wazaglobal also available at: www.waza.org WAZA Membership Printed on FSC-certified paper WAZA members as of 24 October 2019 Affiliates 10 Associations 24 Corporates 19 Institutions 282 Future Events 2020: WAZA/IZE Annual Conference, San Diego Zoo, San Diego, USA 11-15 October Cover Photo: Young chimpanzee : Moscow Zoo, Moscow, Russia rescued from the illegal wildlife trade. 2021 ©️ Pan African Sanctuary Alliance (PASA) 2022: Loro Parque, Tenerife, Spain President’s Letter Jenny Gray WAZA President July 2017 - November 2019 It is hard to isolate a single threat as contributing to the local extinction of species in the wild. Most often, various different threats overlap, each one compounding the negative impacts and ultimately resulting in the collapse of a species. Yet, I can’t help thinking that the illegal removal and trade of animals must be the most evil of the contributing factors. -

Remembering Dr. Arthur Crane Risser by Josef Lindholm III, It Stayed That Way Through the Rest of the 1960’S
Remembering Dr. Arthur Crane Risser by Josef Lindholm III, it stayed that way through the rest of the 1960’s. On Dec. 31, Senior Aviculturist, The Dallas World Aquarium 1969, it reached an all-time high of 1,126 species and subspecies of birds (and 3,465 specimens). Then it dropped. At the end of Art Risser’s death following a stroke on the day after Christ- 1970 there were 1,097 taxa. On Jan. 1, 1972, there were 917. A mas 2008, was entirely unexpected. But many of his saddened year later there were 856. And on Jan1., 1974, the number stood friends were also startled to learn he was 70. I think most of us at 772. I found this deeply disturbing. thought he was far younger. When I first met him, shortly after At the same time, my own small avicultural world had also his arrival at the San Diego Zoo, as Assistant Curator of Birds, become much smaller. In 1972, I was, with much effort, able to in 1974, I thought he was in his late twenties. He was, in fact, 35 convince my parents to buy me Red-eared Waxbills at Wool- when he thus entered the zoo profession, having previously been worth’s and Strawberry Finches and Cut-throats at the White involved in mammalogy. Front, all for $3.95 a pair. In 1974, I found the prices for all of He earned his Master’s in Wildlife Management from the these were now $40 a pair. University of Arizona, in 1963, conducting field research on In answer to the question that all young zoo enthusiasts ask: White-nosed Coatis. -

Academy of Natural Sciences of Drexel Uni- Altissima, Ailanthus 48, 49, 57, 62, 181, 182 Versity 45, 77, 89, 111, 151, 178, 198, Amaranthus 26 247, 276
alkaloids 293 Allee effects 178, 179 Index Allee, Warder Clyde 172, 178 Allegheny River 301 A Allentown, Pennsylvania 271, 272, 274 altissima, Ageratina 55 Academy of Natural Sciences of Drexel Uni- altissima, Ailanthus 48, 49, 57, 62, 181, 182 versity 45, 77, 89, 111, 151, 178, 198, Amaranthus 26 247, 276. See also Academy of Natural Amaranthus hybridus 184 Sciences of Philadelphia Amaranthus retroflexus 180 Academy of Natural Sciences of Philadelphia 2, Ambigolimax valentianus 172 35, 48, 53, 68, 78, 87, 111, 118, 155, 164, Ambrosia 26 168, 179, 206, 208, 212, 216, 230, 300 Ambrosia artemisiifolia 203, 296 Acanalonia conica 228 Ameiurus catus 216 Acanthocephala 168–170 Ameiurus nebulosus 215–226. See also brown “An Account of the Crustacea of the United bullhead catfish States” 164 americana, Malacosoma 51 Acer 78 americana, Phytolacca 140–142 acerifolia, Platanus 5 americana, Setophaga [Parula] 67–74. See Acer negundo 314 also parula warblers Acer palmatum 41 American Civic Association 243 Acer platanoides 247 American crow 150 acid rain 299 American Ornithologist’s Union 70 Acrelius, Israel 128, 130 American Ornithology 9, 140 adventitium, Bipalium 136 American Philosophical Society of Philadelphia Aedes aegypti 307 48, 174, 238 Aedes albopictus 307 American robin 138, 139–149, 298, 301, 303, aegypti, Aedes 307 306 Aesculus sp. 16 American Spiders and Their Spinning Work 96 Ageratina altissima 55 American toad 230 aggregation behavior americanus, Bufo 230 agricultural pests 274, 275. See also specific American Water Works Association 301 pests Ampelopsis brevipedunculata 117, 123 Ailanthus altissima 48, 49, 57, 62, 181, 182 Amphibia 230 ailanthus silkmoth 45–52, 316 amphibians, urban dryness and 230–231 ailanthus tree 20, 28, 46–47, 50, 53, 57, 62, 63, Amphidasys betularia 245. -

Pheasants by Mary Dam Haines City, Florida
Bulwer's pheasant, Lophura bu/wen·. Temminck Tragopan, Tragopan temmincki. Golden pheasant, Chrysolophus pictus. Burmese grey peacock pheasant, Polyplectron bicalcaratum bicalcaratum 8 Pheasants by Mary Dam Haines City, Florida t he propagating of pheasants has been may form close ties. However, pheasants a happy experience for our family for 15 generally do not form a bond as do many years. At TruLi D Farm we raise phea other birds. sants, ducks, geese, swans and assorted 3. It is interesting to note that polygamy or other birds. It is a family project and is so loose association exists among birds of all varied to accommodate the interests of all orders in which the male shows a highly family members. We began this avicul specialized and ornamental plummage. ~ tural hobby with pheasants, and these in 4. There is a great variation in male court teresting birds continue to be the most ship display. Some of the more colorful We challenging and fascinating of all the birds birds are magnificent with a projecting of Buy Birds to us. We began our collection with the horns from the head and an extension of most common of pheasants and kept add wattles all the way to the ground. Some of We Sh1p Birds ing to the number until we, at one time or the pheasants use foot stomping and COMPLETE BIRD SUPPLIES another, have kept most of the species. As emit a piercing scream which can be heard we matured in our expertise, and more im for miles. (714) 527-3387 portantly in our thinking, we finally settled 5. -

Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation ProjectTurtles of the IUCN/SSC of the World Tortoise – 2012and Freshwater Checklist Turtle Specialist Group 000.243 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.000.checklist.v5.2012 © 2012 by Chelonian Research Foundation • Published 31 December 2012 Turtles of the World, 2012 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status TUR T LE TAXONOMY WORKING GROUP * *Authorship of this article is by this working group of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, which for the purposes of this document consisted of the following contributors: PE T ER PAUL VAN DIJK 1, JOHN B. IVERSON 2, H. BRA D LEY SHAFFER 3, ROGER BOUR 4, AN D AN D ERS G.J. RHO D IN 5 1Co-Chair, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, Virginia 22202 USA [[email protected]]; 2Department of Biology, Earlham College, Richmond, Indiana 47374 USA [[email protected]]; 3Department of Ecology and Evolutionary Biology and Institute of the Environment and Sustainability, University of California, Los Angeles, California 90095 USA [[email protected]]; 4Laboratoire des Reptiles et Amphibiens, Muséum National d’Histoire Naturelle, Paris, France [[email protected]]; 5Chairman Emeritus, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Chelonian Research Foundation, 168 Goodrich St., Lunenburg, Massachusetts 01462 USA [[email protected]] AB S T RAC T .