The Genetics of Shortleaf Pine (Pinus Echinata Mill.) with Implications for Restoration and Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Native Woody Plants of Montgomery County, Maryland
Native Woody Plants of Montgomery County, Maryland ~ John Mills Parrish, 2002 Plant List State Where Latin Name Common Name Rank/Status Occurrence Found GYMNOSPERMAE - GYMNOSPERMS Cupressaceae - Cypress Family Juniperus Red Cedar C virginiana Pinaceae - Pine Family Pinus strobus White Pine VR Patuxent St. Park; Northwest Br. Park Pinus rigida Pitch Pine UC Scattered throughout county Pinus echinata Yellow/Shortleaf Pine UC Scattered throughout county Pinus pungens Table-mountain Pine VR NW Branch Pk; Blockhouse Pt. Park Pinus Virginia Pine C virginiana Tsuga Hemlock VR Patuxent St. canadensis Pk; Seneca Ck. St. Park ANGIOSPERMAE - MONOCOTS Smilacaceae - Catbrier Family Smilax glauca Glaucous Greenbrier C Smilax hispida Bristly Greenbrier UC/R Potomac (syn. S. River & Rock tamnoides) Ck. floodplain Smilax Common Greenbrier C rotundifolia ANGIOSPERMAE - DICOTS Salicaceae - Willow Family Salix nigra Black Willow C Salix Carolina Willow S3 R Potomac caroliniana River floodplain Salix interior Sandbar Willow S1/E VR/X? Plummer's & (syn. S. exigua) High Is. (1902) (S.I.) Salix humilis Prairie Willow R Travilah Serpentine Barrens Salix sericea Silky Willow UC Little Bennett Pk.; NW Br. Pk. (Layhill) Populus Big-tooth Aspen UC Scattered grandidentata across county - (uplands) Populus Cottonwood FC deltoides Myricaceae - Bayberry Family Myrica cerifera Southern Bayberry VR Little Paint Branch n. of Fairland Park Comptonia Sweet Fern VR/X? Lewisdale, peregrina (pers. com. C. Bergmann) Juglandaceae - Walnut Family Juglans cinerea Butternut S2S3 R -

Pinus Echinata Shortleaf Pine
PinusPinus echinataechinata shortleafshortleaf pinepine by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia One of the most widespread pines of the Eastern United Sates is Pinus echinata, shortleaf pine. Shortleaf pine was identified and named in 1768. The scientific name means a “prickly pine cone tree.” Other common names for shortleaf pine include shortstraw pine, yellow pine, Southern yellow pine, shortleaf yellow pine, Arkansas soft pine, Arkansas pine, and old field pine. Among all the Southern yellow pines it has the greatest range and is most tolerant of a variety of sites. Shortleaf pine grows Southeast of a line between New York and Texas. It is widespread in Georgia except for coastal coun- ties. Note the Georgia range map figure. Pinus echinata is found growing in many mixtures with other pines and hardwoods. It tends to grow on medium to dry, well-drained, infertile sites, as compared with loblolly pine (Pinus taeda). It grows quickly in deep, well-drained areas of floodplains, but cannot tolerate high pH and high calcium concentrations. Compared with other Southern yellow pines, shortleaf is less demanding of soil oxygen content and essential element availability. It grows in Hardiness Zone 6a - 8b and Heat Zone 6-9. The lowest number of Hardiness Zone tends to delineate the Northern range limit and the largest Heat Zone number tends to define the South- ern edge of the range. This native Georgia pine grows in Coder Tree Grow Zone (CTGZ) A-D (a mul- tiple climatic attribute based map), and in the temperature and precipitation cluster based Coder Tree Planting Zone 1-6. -

Important Food Plants for Backyard Songbirds of the Catskills
Important Food Plants for Backyard Songbirds of the Catskills Woody Plants ****25-50%, ***10-25% of diet **5-10%, *2-5&% of diet 0.5-2% of diet Maples Box-elder Acer negundo Evening Grosbeak **** Coccothraustes vespertinus American Goldfinch Carduelis tristis Moosewood Acer pensylvanicum Purple Finch * Carpodacus purpureus Yellow-bellied Sapsucker (sap) Sphyrapicus varius Red Maple Acer rubrum Rose-breasted Grosbeak * Pheucticus ludovicianus Silver Maple Acer saccharinum Red-breasted Nuthatch * Sitta canadensis Sugar Maple Acer saccharum Mountain Maple Acer spicatum Serviceberries Downy Serviceberry Amelanchier arborea Cedar Waxwing * Bombicilla cedrorum Tufted Titmouse Baeolophus bicolor Shadblow Serviceberry Amelanchier canadensis Veery * Catharus fuscescens Northern Cardinal Cardinalis cardinalis Smooth Serviceberry Amelanchier laevis Hermit Thrush * Catharus guttatus Hermit Thrush Catharus guttatus Running Serviceberry Amelanchier stolonifera Gray Catbird * Dumetella carolinensis Northern Flicker Coraptes auratus Baltimore Oriole * Icterus galbula American Crow Corvus brachyrhyncos Blue Jay Cyanocitta cristata Wood Thrush Hylocichla mustelina Northern Mockingbird Mimus polyglottus Eastern Towhee Papilo erythrophthalmus Rose-breasted Grosbeak Pheucticus ludovicianus Downy Woodpecker Picoides pubescens Hairy Woodpecker Picoides villosus Scarlet Tanager Piranga olivacea Black-capped Chickadee Poecile atricapillus Eastern Bluebird Sialia sialis Brown Thrasher Toxostoma rufin American Robin Turdus migratorius Aralias Bristly Sarsparilla -

Introgression Between Pinus Taeda L. and Pinus Echinata Mill
Introgression between Pinus taeda L. and Pinus echinata Mill. Jiwang Chen 1, C. G. Tauer l , Guihua Bai1, and Yinghua Huang1 Key words: cpDNA; introgression; microsatellite; nuclear ribosomal DNA; loblolly pine; shortleaf pine INTRODUCTION Loblolly pine (Pinus taeda L.) and shortleaf pine (Pinus echinata Mill.) have widely overlapping geographic ranges. Hybridization between the two species has interested tree breeders for a long time. Morphologically, the two pine species are different. The needles of loblolly pine are 6 to 9 inches long, usually with three yellow-green needles per fascicle; but shortleaf pine needles are 3 to 5 inches long, with two or three dark yellow- green slender and flexible needles per fascicle. Loblolly pine also has larger cones than shortleaf, as well as other differences, however, these characters offer limited help when the genotypes of the parents and their probable hybrids are compounded by environmental factors The limitations of morphological characters resulted in the identification of the allozyme marker IDH (isocitrate dehydrogenase) to identify hybrids (Huneycutt and Askew, 1989). The high frequency of IDH variation seen in natural shortleaf pine populations outside the natural range of loblolly pine (Rajiv et al., 1997) suggests either profuse hybridization between the two species or that IDH is an unreliable marker. These data required us to look for new markers to confirm the identity of putative hybrids. A more extensive study (relative to the study of Rajiv et al., 1997), sampling a larger portion of shortleaf-loblolly pine sympatric population, was conducted to further explore the nature and extent of these hybrids in the native populations. -
![SHORTLEAF PINE [Pinaceae] Pinus Echinata Miller](https://docslib.b-cdn.net/cover/6490/shortleaf-pine-pinaceae-pinus-echinata-miller-586490.webp)
SHORTLEAF PINE [Pinaceae] Pinus Echinata Miller
Vascular Plants of Williamson County Pinus echinata − SHORTLEAF PINE [Pinaceae] Pinus echinata Miller (possible selection, mixed-sized plants in woodland of escaped plants originating from cultivated specimens), SHORTLEAF PINE. Tree, evergreen, with 1 trunk to 30 cm diameter (non-cultivated), in range 4−10 m tall (reproductive); monoecious; shoots with long shoot-short shoot organization, long shoot growth beginning in late March, with closely spaced, nonphotosynthetic scale leaves along new axis (new spring growth after pollination begins), springtime long shoot initially to 200 × 8 mm before foliage leaf elongating, at each node having a scalelike primary leaf but immediately producing a short shoot in the axil of scale leaf (sylleptic development) = a “fascicle” of 2−3 photosynthetic leaves (needles) held together by several tightly wrapped papery (scarious) leaves at the base, glabrous, having resin ducts within plant, aromatic, especially when crushed or damaged. Stems: internodes relatively short hidden by persistent bases of scale leaves; young twigs flexible, lacking leaves when > 4 mm diameter, with helically arranged remnants of scale leaves; older stems with woody seed cones mostly 7−8 mm diameter shedding tannish gray leaf bases and forming light brown periderm; bark large- scaly, not peeling and tightly attached, gray, not obviously resinous. Leaves: of 3 types (scale, foliage, wrapper); scale leaves on new long shoots helically alternate, simple, sessile with decurrent bases; foliage leaves (needles) terminal and mostly -

Glenn Oakes Bioblitz Report
Glen Oakes BioBlitz Report Note: The black line in the map denotes Glen Oakes Town Forest in Fremont, N.H. From May, 2011 Glenn Oakes Bioblitz 2011 page 2 of 11 whk Introduction On and around the weekend of May 21, 2011, an intensive species inventory (BioBlitz) was made in Fremont, NH within the Glen Oakes Town Forest. The event was organized to enhance the baseline information of the biodiversity found in this area begun by Forester Charles A. Moreno. Three small teams of volunteers lead by experts spent the morning of Saturday, May 21, 2011 scouring the Glen Oakes Town Forest in three different directions keeping an inventory of the species they identified. In addition, other volunteers unable to attend the event that day reported their findings from the week before and after the event. The result of these efforts is the species inventory list found in the right-hand column (verified 5/2011) of this report. The third column from the left contains observations made by Charles A. Moreno during the period of time he was working on the Forest Management Plan for the Glen Oakes Conservation Area (2009). Together the two columns represent the observed biodiversity of this area This conservation area, a part of the larger Spruce Swamp ecosystem, provides a unique haven of biodiversity. Much of the area inventoried has been identified as “highest quality habitat” or as important “supporting habitat” in the New Hampshire Wildlife Action Plan – please see the map printed on the cover of this report. It is worth noting the area contains the critical habitats required for several Species of Greatest Conservation Concern in New Hampshire. -

Pinus Echinata)-Hardwood Mixtures in Low Quality Mixed Upland Hardwood Stands Using Cluster Planting and Natural Regeneration
Article Restoration of Shortleaf Pine (Pinus echinata)-Hardwood Mixtures in Low Quality Mixed Upland Hardwood Stands Using Cluster Planting and Natural Regeneration David Clabo 1,* and Wayne Clatterbuck 2 1 Warnell School of Forestry & Natural Resources, University of Georgia, 4601 Research Way, Tifton, GA 31793, USA 2 Department of Forestry, Wildlife & Fisheries, University of Tennessee, 274 Ellington Plant Sciences Building, Knoxville, TN 37996, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-229-386-3672 Received: 3 April 2020; Accepted: 15 April 2020; Published: 17 April 2020 Abstract: Cluster planting of shortleaf pine, along with various site preparation and release treatments, were tested to restore mixed shortleaf pine (Pinus echinata Mill.)–hardwood stands in areas where the shortleaf pine has diminished in recent years. Shortleaf pine–hardwood mixtures were once a common forest type throughout the Cumberland Mountains and Plateau physiographic regions of the southeastern United States. Knowledge of how to restore shortleaf pine–hardwood mixtures is limited throughout shortleaf pine’s large native range. The objectives of this study were to compare planted shortleaf pine and natural hardwood regeneration survival, growth, and composition following various site preparation and early release treatments. Cluster planting and partial timber harvesting were used to reintroduce shortleaf pine and create two-aged stands in the Cumberland Mountains of Tennessee, USA. Results indicated that shortleaf pine survival, basal diameter, and height growth did not differ following four growing seasons among treatments. Natural regeneration stem densities and heights within shortleaf pine clusters did not differ significantly by treatment. Natural regeneration stem densities differed by species group and height class across the site, while the treatment species interaction term was also significant. -
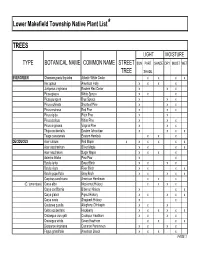
Native Plant List Trees.XLS
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Hands Creek Farm Nature Preserve Management Plan
Hands Creek Farm Nature Preserve Management Plan Prepared by A. Gaites/Land Acquisition & Management Dept. with assistance from L. D’Andrea and T. Borsack/Planning Dept. December 2009 Adopted by Resolution 2011-96; January 20, 2011 I. Introduction The Hands Creek Farm Nature Preserve is made up of eight reserved area parcels situated between Hands Creek Road and Middle Highway in the East Hampton School District. This plan will cover the following parcels: Suffolk County Tax Map # Acreage 300-118-1-12.65 1.9 300-118-1-12.66 1.8 300-118-1-12.67 12.8 300-118-1-12.68 14.2 300-118-1-12.69 2.1 300-118-1-12.70 0.7 300-118-1-57.13 7.1 300-118-1-57.14 0.5 These properties were acquired by the Town of East Hampton as a gift from the Hands Creek Farm Property Owner’s Association, Inc. for the purposes of open space preservation. Resolution #166, authorizing this acquisition was passed on January 18, 2001. The resolution references some significant natural features including a kettle hole, trail corridor, road buffers, and an unusual open meadow. The properties were designated as Nature Preserves on November 7, 2003 with resolution #1469. II. Description of Site The winding, irregular shapes of these reserved area parcels is best understood by viewing an aerial photograph overlaid with Suffolk County Tax Map lines (see Appendix IV). The majority of the Hands Creek Nature Preserve is wooded, consisting of pitch pine (Pinus rigida) and oak (Quercus sp.) trees. -

Pinus Echinata, Shortleaf Pine
PinusPinus echinataechinata shortleafshortleaf pinepine by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia One of the most widespread pines of the Eastern United Sates is Pinus echinata, shortleaf pine. Shortleaf pine was identified and named in 1768. The scientific name means a “prickly pine cone tree.” Other common names for shortleaf pine include shortstraw pine, yellow pine, Southern yellow pine, shortleaf yellow pine, Arkansas soft pine, Arkansas pine, and old field pine. Among all the Southern yellow pines it has the greatest range and is most tolerant of a variety of sites. Shortleaf pine grows Southeast of a line between New York and Texas. It is widespread in Georgia except for coastal coun- ties. Note the Georgia range map figure. Pinus echinata is found growing in many mixtures with other pines and hardwoods. It tends to grow on medium to dry, well-drained, infertile sites, as compared with loblolly pine (Pinus taeda). It grows quickly in deep, well-drained areas of floodplains, but cannot tolerate high pH and high calcium concentrations. Compared with other Southern yellow pines, shortleaf is less demanding of soil oxygen content and essential element availability. It grows in Hardiness Zone 6a - 8b and Heat Zone 6-9. The lowest number of Hardiness Zone tends to delineate the Northern range limit and the largest Heat Zone number tends to define the South- ern edge of the range. This native Georgia pine grows in Coder Tree Grow Zone (CTGZ) A-D (a mul- tiple climatic attribute based map), and in the temperature and precipitation cluster based Coder Tree Planting Zone 1-6. -

Pinus Echinata Mill.) AND
DYNAMICS OF UNDERTORY SHORTLEAF PINE (Pinus echinata Mill.) AND HARDWOOD AFTER THINNING SHORTLEAF PINE FORESTS IN THE SOUTHEASTERN UNITED STATES By ANUP KC Bachelor of Science in Forestry Institute of Forestry, TU Pokhara, Nepal 2007 Master of Science in Aquaculture and Fisheries University of Arkansas at Pine Bluff Pine Bluff, Arkansas 2011 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 2016 DYNAMICS OF UNDERTORY SHORTLEAF PINE (Pinus echinata Mill.) AND HARDWOOD AFTER THINNING SHORTLEAF PINE FORESTS IN THE SOUTHEASTERN UNITED STATES Dissertation Approved: Thomas B. Lynch Dissertation Advisor Lan Zhu Rod Will Stephen Hallgren ii ACKNOWLEDGEMENTS I would like to express my sincere thanks to my major advisor, Dr. Thomas B. Lynch, for his guidance and support during the last four years of my research. I am thankful to Dr. Rodney Will, Dr. Lan Zhu, and Dr. Stephen Hallgren for serving on my dissertation committee. I am also thankful to the Department of Natural Resource Ecology and Management for funding my research and education for four years. I am especially thankful to my family. I would like to thank my father and mother for everything that they have done for me in this life. Thank you to my lovely wife, Ambika Shrestha, for her hard work, patience, and the encouragement throughout this journey. I thank my daughter Ansu for being the most adorable little girl I have ever encountered. I also want to express my sincere thanks to my uncles and aunts for their unwavering love and support. -

The Importance of Shortleaf Pine for Wildlife and Diversity in Mixed Oak-Pine Forests and in Pine-Grassland Woodlands
THE IMPORTANCE OF SHORTLEAF PINE FOR WILDLIFE AND DIVERSITY IN MIXED OAK-PINE FORESTS AND IN PINE-GRASSLAND WOODLANDS Ronald E. Masters1 ABSTRACT.—Shortleaf pine, by virtue of its wide distribution and occurrence in many forest types in eastern North America, is an important species that provides high habitat value for many wildlife species. Shortleaf pine functions as a structural habitat element in both mixed oak-pine forests and in pine-grassland woodlands. It also adds diversity throughout all stages of plant succession and stand development. Within the range of shortleaf pine, wildlife species are variously associated with shortleaf based on stand density, the proportion of hardwoods within a structural stage of development, and availability of habitat structure within the specifi c niche that each wildlife species occupies. Shortleaf also is a key species in ecosystems where it occurs naturally because its occurrence and relative dominance are defi ned to a large extent by the natural disturbance regime, particularly fi re. Fire frequency and season, to some extent, defi ne the understory plant community response and determine shortleaf pine’s potential for regeneration, establishment of future codominant and dominant trees, and perpetuate a relative mix of pines with other associated tree species within a stand. This understory community response to fi re or lack of fi re defi nes much of the ground-dwelling or ground-foraging wildlife species populations. This paper discusses wildlife species associated with different structural characteristics and fi re regime in mixed oak-shortleaf and shortleaf- dominated forests and woodlands. INTRODUCTION stands (Turner 1935, Oosting 1942).