The Study of Leukocyte Phagocytic Activity in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHITE BLOOD CELLS Formation Function ~ TEST YOURSELF
Chapter 9 Blood, Lymph, and Immunity 231 WHITE BLOOD CELLS All white blood cells develop in the bone marrow except Any nucleated cell normally found in blood is a white blood for some lymphocytes (they start out in bone marrow but cell. White blood cells are also known as WBCs or leukocytes. develop elsewhere). At the beginning of leukopoiesis all the When white blood cells accumulate in one place, they grossly immature white blood cells look alike even though they're appear white or cream-colored. For example, pus is an accu- already committed to a specific cell line. It's not until the mulation of white blood cells. Mature white blood cells are cells start developing some of their unique characteristics larger than mature red blood cells. that we can tell them apart. There are five types of white blood cells. They are neu- Function trophils, eosinophils, basophils, monocytes and lymphocytes (Table 9-2). The function of all white blood cells is to provide a defense White blood cells can be classified in three different ways: for the body against foreign invaders. Each type of white 1. Type of defense function blood cell has its own unique role in this defense. If all the • Phagocytosis: neutrophils, eosinophils, basophils, mono- white blood cells are functioning properly, an animal has a cytes good chance of remaining healthy. Individual white blood • Antibody production and cellular immunity: lympho- cell functions will be discussed with each cell type (see cytes Table 9-2). 2. Shape of nucleus In providing defense against foreign invaders, the white • Polymorphonuclear (multilobed, segmented nucleus): blood cells do their jobs primarily out in the tissues. -

Automatic System for Differential Blood Counting
ISSN (Print) : 2320 – 3765 ISSN (Online): 2278 – 8875 International Journal of Advanced Research in Electrical, Electronics and Instrumentation Engineering (An ISO 3297: 2007 Certified Organization) Vol. 5, Issue 4, April 2016 Automatic System for Differential Blood Counting 1 2 Manisha Shirvoikar , Dr.H.G.Virani PG Student [ECI], Dept. of ETC, Goa College of Engineering, Ponda, Goa, India1 Professor & Head of Department, Dept. of ETC, Goa College of Engineering, Ponda, Goa, India2 ABSTRACT: For detecting various diseases, Doctor first suggests the patient to undergo blood test which is used as a health indicator. Differential Blood Count (DBC) provides haematologist with valuable information about health of the patient. DBC determines the percentage of types of WBC this is important because it give exact count of five types of WBC such as neutrophil, lymphocyte, monocyte, eosinophil and basophile. Increase or decrease of DBC than the ideal count indicated that our body is not healthy. Precise counting of type of WBC is very important. Manual counting of White blood cells is time consuming and can lead to human error with increase in number of samples. Automatic cell counter sometimes misclassifies the cells having different morphology. Even they are very expensive and unaffordable by remote area health centres and hospitals. These problems are overcome by developing a system which is image based, cost effective, fast and accurate which has the capability to identify, classify the different type of white blood cell and perform DBC. Implementation is done using MATLABR2014b. KEYWORDS: MATLAB, peripheral blood smear, RBC, Thresholding, WBC, DBC. I.INTRODUCTION Total volume of blood in human is 5-6 litres i.e 8% of body weight or 80 mL/kg body weight. -

Digitalcommons@UNMC Granulocytopenia
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1936 Granulocytopenia Howard E. Mitchell University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Mitchell, Howard E., "Granulocytopenia" (1936). MD Theses. 457. https://digitalcommons.unmc.edu/mdtheses/457 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. G PA~lULOCYTOPENI A SENIOR THESIS By Howard E. Mitchell April 17, 1936 TABLE OF CONT'ENTS Introduction Definition • · 1 History . • • • 1 Nomenclature • • • • • 4 ClassificBtion • • • • 6 Physiology • • • • .10 Etiology • • 22 Geographic Distribution • 23 Age, Sex, and R9ce • • ·• 23 Occupation • .. • • • • .. • 23 Ba.cteria • • • • .. 24 Glandu18.r Dysfunction • • • 27 Radiation • • • • 28 Allergy • • • 28 Chemotactic and Maturation Factors • • 28 Chemicals • • • • • 30 Pathology • • • • • 36 Symptoms • • • • • • • 43 DiEtgnosis • • • • • .. • • • • • .. • 4'7 Prognosis 48 '" • • • • • • • • • • • • Treatment • • • • • • • • 49 Non"'specific Therapy • • • • .. 50 Transfusion • • • • .. 51 X-Ray • • • • • • • • • 52 Liver ·Extract • • • • • • • 53 Nucleotides • • • • • • • • • • • 53 General Ca.re • • • • • • • • 57 Conclusion • • • • • • • • • 58 480805 INTHODUCTION Although t~ere is reference in literature of the Nineteenth Century to syndromes similating the disease (granulocytopenia) 9.8 W(~ know it todes, it "vas not un til the year 1922 that Schultz 8ctually described his C8se as a disease entity and by so doing, stimulated the interest of tne medical profession to further in vestigation. -

Ingenuity Pathway Analysis of Human Facet Joint Tissues: Insight Into Facet Joint Osteoarthritis
EXPERIMENTAL AND THERAPEUTIC MEDICINE 19: 2997-3008, 2020 Ingenuity pathway analysis of human facet joint tissues: Insight into facet joint osteoarthritis CHU CHEN*, SHENGYU CUI*, WEIDONG LI, HURICHA JIN, JIANBO FAN, YUYU SUN and ZHIMING CUI Department of Spine Surgery, The Second Affiliated Hospital of Nantong University, Nantong, Jiangsu 226001, P.R. China Received August 17, 2019; Accepted January 30, 2020 DOI: 10.3892/etm.2020.8555 Abstract. Facet joint osteoarthritis (FJOA) is a common the top 5 IPA networks (with a score >30). The present study degenerative joint disorder with high prevalence in the elderly. provides insight into the pathological processes of FJOA from FJOA causes lower back pain and lower extremity pain, and a genetic perspective and may thus benefit the clinical treat- thus severely impacts the quality of life of affected patients. ment of FJOA. Emerging studies have focused on the histomorphological and histomorphometric changes in FJOA. However, the dynamic Introduction genetic changes in FJOA have remained to be clearly deter- mined. In the present study, previously obtained RNA deep Facet joint osteoarthritis (FJOA) is a common degenerative sequencing data were subjected to an ingenuity pathway joint disorder that causes the degeneration and breakdown analysis (IPA) and canonical signaling pathways of differ- of cartilage and restricts the movement of joints (1). It has entially expressed genes (DEGs) in FJOA were studied. The been reported that lumbar FJOA occurs at high prevalence top 25 enriched canonical signaling pathways were identified and the presence of FJOA is highly associated with age (2,3). and canonical signaling pathways with high absolute values A community-based cross-sectional study indicated that of z-scores, specifically leukocyte extravasation signaling, in populations aged <50 years, the prevalence of FJOA was Tec kinase signaling and osteoarthritis pathway, were inves- <45%, while it was ~75% in populations aged >50 years (4). -

Simplified White Blood Cell Differential: an Inexpensive, Smartphone- and Paper-Based Blood Cell Count
Simplified White Blood Cell Differential: An Inexpensive, Smartphone- and Paper-Based Blood Cell Count Item Type Article Authors Bills, Matthew V.; Nguyen, Brandon T.; Yoon, Jeong-Yeol Citation M. V. Bills, B. T. Nguyen and J. Yoon, "Simplified White Blood Cell Differential: An Inexpensive, Smartphone- and Paper-Based Blood Cell Count," in IEEE Sensors Journal, vol. 19, no. 18, pp. 7822-7828, 15 Sept.15, 2019. doi: 10.1109/JSEN.2019.2920235 DOI 10.1109/jsen.2019.2920235 Publisher IEEE-INST ELECTRICAL ELECTRONICS ENGINEERS INC Journal IEEE SENSORS JOURNAL Rights © 2019 IEEE. Download date 25/09/2021 12:19:07 Item License http://rightsstatements.org/vocab/InC/1.0/ Version Final accepted manuscript Link to Item http://hdl.handle.net/10150/634549 > REPLACE THIS LINE WITH YOUR PAPER IDENTIFICATION NUMBER (DOUBLE-CLICK HERE TO EDIT) < 1 Simplified White Blood Cell Differential: An Inexpensive, Smartphone- and Paper-Based Blood Cell Count Matthew V. Bills, Brandon T. Nguyen, and Jeong-Yeol Yoon or trained lab specialist to prepare blood smear slides, stain Abstract— Sorting and measuring blood by cell type is them, and then manually count different WBC types using a extremely valuable clinically and provides physicians with key hemocytometer under a microscope [3]. To do this they must information for diagnosing many different disease states dilute specimens in a red blood cell (RBC) lysing solution to including: leukemia, autoimmune disorders, bacterial infections, remove RBCs and count WBCs. Manually counting WBCs is etc. Despite the value, the present methods are unnecessarily laborious and requires specialized medical equipment and costly and inhibitive particularly in resource poor settings, as they require multiple steps of reagent and/or dye additions and trained personnel. -

Rheumatoid Arthritis Synovial Fluid Neutrophils Drive Inflammation
medRxiv preprint doi: https://doi.org/10.1101/2020.07.16.20155291; this version posted July 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC 4.0 International license . Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species and neutrophil extracellular traps Helen L Wright 1;∗, Max Lyon 1, Elinor A Chapman 1, Robert J Moots 2, Steven W Edwards 3 1Institute of Life Course and Medical Sciences, University of Liverpool, Liverpool, Merseyside, United Kingdom 2Clinical Sciences Centre, University Hospital Aintree, Liverpool, Merseyside, United Kingdom 3Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, Merseyside, United Kingdom Correspondence*: Dr Helen Wright, University of Liverpool, Institute of Life Course and Medical Sciences, William Henry Duncan Building, 6 West Derby Street, Liverpool, L7 8TX [email protected] ABSTRACT Rheumatoid arthritis (RA) is a chronic inflammatory disorder affecting synovial joints. Neutrophils are believed to play an important role in both the initiation and progression of RA, and large numbers of activated neutrophils are found within both synovial fluid (SF) and synovial tissue from RA joints. In this study we analysed paired blood and SF neutrophils from patients with severe, active RA (DAS28> 5:1, n=3) using RNA-seq. 772 genes were significantly different between blood and SF neutrophils. IPA analysis predicted that SF neutrophils had increased expression of chemokines and ROS production, delayed apoptosis, and activation of signalling cascades regulating the production of NETs. -
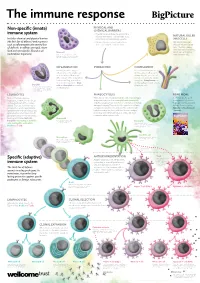
Non-Specific (Innate) Immune System Specific (Adaptive) Immune System
The immune response PHYSICAL AND Non-specific (innate) CHEMICAL BARRIERS immune system • Physical barriers include the skin and the mucous membranes of the airways, guts, NATURAL KILLER Includes chemical and physical barriers and urinary and reproductive systems. (NK) CELLS (the first line of defence) and responses • Chemical barriers include hydrochloric NK cells kill pathogen- such as inflammation (the second line acid secreted by the stomach lining. infected cells and cancer of defence). Its effects are rapid, short- cells. They also release chemicals called cytokines, lived and non-specific. Found in all Mast cell which alert and attract multicellular organisms. Cells involved in allergic responses, releasing other immune cells. histamine and other inflammatory molecules. Mast cells sit within skin and mucosal tissues. INFLAMMATION IF BREACHED COMPLEMENT Invading microbes trigger A set of around 30 proteins in inflammation. This involves an the blood plasma that can be increase in blood flow to the activated by the presence of affected part of the body, which microbes or antibody–antigen leads to swelling, pain and an complexes. Complement can increase in temperature. Mast destroy pathogens and activate Basophil cells and basophils are involved phagocytic cells. Cells involved in allergic and inflammatory responses. Basophils release histamine in inflammation. like mast cells, but unlike mast cells they circulate in the blood. LEUKOCYTES PHAGOCYTOSIS READ MORE Made in the bone marrow, White blood cells including dendritic cells, macrophages Big Picture is a free post- leukocytes, or white blood cells, are and granulocytes such as eosinophils and neutrophils 16 magazine for teachers an important part of the immune engulf (or phagocytose) microbes or cells that are infected, that explores issues around system. -

Effects of Cadmium on Haemopoiesis in Irradiated and Non- Irradiated Mice: 2
Physiol. Res. 45:101-106, 1996 Effects of Cadmium on Haemopoiesis in Irradiated and Non- Irradiated Mice: 2. Relationship to the Number of Circulating Blood Cells and Haemopoiesis N.O. MACKOVÁ, S. LENÍKOVÁ, P. FEDOROČKO, P. BREZÁNI1, A. FEDOROČKOVÁ2 Department of Cellular and Molecular Biology, Faculty of Sciences, department of Medical Biology, Faculty of Medicine, Šafárik University and 2Department of Chemistry, Faculty of Metallurgy, Technical University, Kosice, Slovak Republic Receded September 22, 1995 Accepted December 12, 1995 Summary The effect of administration of cadmium alone in non-irradiated mice as well as the effect of pre irradiation administration of cadmium on the reparation processes of haemopoiesis were investigated in mice irradiated by a dose of 7.5 Gy. The pre-irradiation administration of cadmium accelerated the reparation processes in the bone marrow and spleen as well as the number of leukocytes and thrombocytes in the peripheral blood. The administration of cadmium alone caused a temporary weight decrease of the thymus and reduced number of erythrocytes, reticulocytes and haemoglobin values in the peripheral blood. The temporary rapid increase in the number of leukocytes on the 21st day after cadmium administration was investigated. Key words Cadmium - Haemopoiesis - Gamma radiation Introduction et al. 1976) when it is accumulated rapidly (Sakata et al. 1988). The immune system (Dean et al. 1982, Koller The risk factors caused by industrialization are 1980), especially thymocytes (Xu et al. 1989), react very increasing in our environment. Ionizing radiation sensitively to heavy metals. After administration of belongs to the most dangerous types of pollution cadmium, the suppression of lymphocyte proliferation known worldwide. -

Immunology Immunology
IMMUNOLOGY IMMUNOLOGY ONTOGENY OF THE IMMUNE CELL Learning Objective : ONTOGENY OF • Learn the origin and function of cells of the immune system THE IMMUNE • Antigen recognition molecule of the lymphocytes CELL • Understand generation of receptor diversity Content ONTOGENY OF 1. Origin THE IMMUNE 2. Cell • Lymphoid CELL • Myeloid 3. Functions Origin Haematopoiesis involves the production development differentiation and maturation the blood cells( ONTOGENY OF erythrocytes megakaryocytes, and leukocytes) from THE IMMUNE multipotent stem cell The site of haematopoiesis changes during development. CELL Yolk Sac Liver and spleen Bone marrow ONTOGENY OF THE IMMUNE CELL Origin These multipotent stem cells found in the bone marrow have the ability to undergo asymmetric division. One of the two daughter cells will serve to renew the population of stem cells (self renewal), while the other can give rise to common lymphoid progenitor cell to common myeloid ONTOGENY OF progenitor cell. Different ion into various cell will be guided by the various type of cytokines and growth factors. THE IMMUNE Common lymphoid progenitor- T cell, B cell, Natural killer CELL cell Common Myeloid Progenitor- Erythrocytes, Megakaryocytes, Mast cell, Eosinophils, Basophils, Neutrophils, Monocytes and Dendric cell. ONTOGENY OF THE IMMUNE CELL Haematopoiesis Function The white blood cells of both Myeloid and Lymphoid stem cells have specialized function in the body once their differentiation in the bone marrow is complete. Cell of the ONTOGENY OF myeloid lineage, except erythrocytes and megakaryocytes, perform specific stereotypical response and are members THE IMMUNE of the innate branch of the immune response. B lymphocyte and T lymphocyte of lymphoid linage perform CELL focused, antigen specific roles in immunity. -

Red Blood Cells, Platelets
Components of blood Introduction The different components that make up blood. Plasma, white blood cells, red blood cells, platelets. If you prick your finger or scrape your knee, you'll see some droplets of blood form. Just by eye, these droplets may seem to be made of uniform red liquid, similar to food coloring or paint. However, if you were to look under a microscope, you would see that your blood is actually a mixture of liquid and cells. And if you could zoom in even further, you would see that there are also many macromolecules (such as proteins) and ions (such as sodium) floating in the liquid. All of these components are important to the roles blood plays in the body. What is blood? Blood, by definition, is a fluid that moves through the vessels of a circulatory system. In humans, blood includes plasma (the liquid portion), blood cells (which come in both red and white varieties and platelets. 1. Plasma is the main component of blood and consists mostly of water, with proteins, ions, nutrients, and wastes mixed in. Page 1 of 21 2. Red blood cells (erthrocytes) are responsible for carrying oxygen and carbon dioxide. 3. Platelets (thrombocytes) are responsible for blood clotting. 4. White blood cells (Leukocytes) are part of the immune system and function in immune response. Cells make up about 45%percent percent of human blood, while plasma makes up the other 55%percent percent. Plasma Plasma, the liquid component of blood, can be isolated by spinning a tube of whole blood at high speeds in a centrifuge. -

Primary Agranulocytic Angina
University of Nebraska Medical Center DigitalCommons@UNMC MD Theses Special Collections 5-1-1934 Primary agranulocytic angina Howard M. Coe University of Nebraska Medical Center This manuscript is historical in nature and may not reflect current medical research and practice. Search PubMed for current research. Follow this and additional works at: https://digitalcommons.unmc.edu/mdtheses Part of the Medical Education Commons Recommended Citation Coe, Howard M., "Primary agranulocytic angina" (1934). MD Theses. 314. https://digitalcommons.unmc.edu/mdtheses/314 This Thesis is brought to you for free and open access by the Special Collections at DigitalCommons@UNMC. It has been accepted for inclusion in MD Theses by an authorized administrator of DigitalCommons@UNMC. For more information, please contact [email protected]. ~RlMARY) AGRANULOCYTI C ANGINA by Howard Malin Coe t A.B. Presented to the Faculty of the University of Nebraska in partial fulfillment of the requirements for the Degree of Doctor of Medicine From the Medical College University of Nebraska Omaha,Nebraska April 193# PRIMARY AGRANULOCYTI C ANGINA INTRODUCTION Since its original description by Schultz (1) in 1922, agranulocytic angina has come to occupy more and more prominence in the medical field. Today the liter- ature is flooded with a heterogeneous array of case re ports, many theories of etiology, clinical and laboratory manifestations, and biopsy and necropsy findings. Added to this is a confusing terminology, various attempts at classification, diverse forms of treatment, and many arguments both pro and con as to whether or not agranu locytic angina is a disease entity, a group of diseases, or only a symptom complex. -

White Blood Cells
White Blood Cells White blood cells, or leukocytes, are immune cells that are present in the blood. The detailed mechanisms of immune function are covered in the immunity unit, but we will discuss the classes here. There are five common types of leukocytes (and some of those types have subgroups). One way of categorizing these five is by whether or not they contain granules in their cytoplasm when the cells are stained. If they do, they are a type of granulocyte. If not, they fall into the category of agranulocytes. Another way of distinguishing leukocytes from one another is by the morphology (shape and patten) of their nuclei. Granulocytes have multilobulated nuclei and agranulocytes have a spherical (nonlobulated) nucleus. The lifespan of a granulocyte ranges from about 12 hours to 4 days, but the agranulocyte survives for approximately 100 to 300 days. Leukocytes mainly function to protect the body from microbial invasion and toxins. WBCs readily travel through the bloodstream. However, certain leukocytes have the ability to move into the interstitial spaces of the body’s tissues in order to attack foreign agents to protect the body from infection. They leave the capillalry by squeezing through the pores in the capillary walls via a process called diapedesis. This is due to the ability of these leukocytes to rearrange cytoskeletal matrix in a manner that changes their shape. Granulocytes Granulocytes contain many granules within the cytoplasm, and they have a multilobular, irregularly shaped nucleus. There are three types of granulocytes: neutrophils, eosinophils, and basophils. Specific stains used to reveal the granules in their cytoplasm identify each granulocyte.