Neurostimulation for Drug-Resistant Epilepsy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

The Neurobiology of Agrammatic Sentence Comprehension: a Lesion Study
The Neurobiology of Agrammatic Sentence Comprehension: A Lesion Study Corianne Rogalsky1, Arianna N. LaCroix1, Kuan-Hua Chen2,3, Steven W. Anderson2, Hanna Damasio4, Tracy Love5, and Gregory Hickok6 Abstract ■ Broca’s area has long been implicated in sentence compre- average did not exhibit the expected agrammatic compre- hension. Damage to this region is thought to be the central hension pattern—for example, their performance was >80% source of “agrammatic comprehension” in which performance on noncanonical sentences in the sentence–picture matching is substantially worse (and near chance) on sentences with non- task. Patients with ATL damage (n = 18) also did not exhibit canonical word orders compared with canonical word order an agrammatic comprehension pattern. Across our entire sentences (in English). This claim is supported by functional patient sample, the lesions of patients with agrammatic com- neuroimaging studies demonstrating greater activation in prehension patterns in either task had maximal overlap in pos- Broca’s area for noncanonical versus canonical sentences. terior superior temporal and inferior parietal regions. Using However, functional neuroimaging studies also have frequently voxel-based lesion–symptom mapping, we find that lower per- implicated the anterior temporal lobe (ATL) in sentence pro- formances on canonical and noncanonical sentences in each cessing more broadly, and recent lesion–symptom mapping task are both associated with damage to a large left superior studies have implicated the ATL and mid temporal regions in temporal–inferior parietal network including portions of the agrammatic comprehension. This study investigates these ATL, but not Broca’s area. Notably, however, response bias in seemingly conflicting findings in 66 left-hemisphere patients plausibility judgments was significantly associated with damage with chronic focal cerebral damage. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

Where Is the Anterior Temporal Lobe and What Does It Do?
The Journal of Neuroscience, March 6, 2013 • 33(10):4213–4215 • 4213 Journal Club Editor’s Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/site/misc/ifa_features.xhtml. Where Is the Anterior Temporal Lobe and What Does It Do? Michael F. Bonner1 and Amy R. Price1,2 1Department of Neurology and 2Neuroscience Graduate Group, University of Pennsylvania, Philadelphia, Pennsylvania 19104 Review of Peelen and Caramazza The anterior temporal lobe (ATL) is these findings have implicated a large re- represented (Simmons and Barsalou, thought to be critical for semantic memory– gion of the ATL in semantic memory. 2003). This work has implications for our our knowledge of objects, people, words, One might expect that complementary understanding of how ATL structures dif- and facts. However, there is substantial functional neuroimaging studies would ferentially contribute to semantic memory, disagreement over the precise role of the provide a more fine-grained picture of and how the semantic system is shaped ATL in semantic memory, and there is ATL function. Unfortunately, the evi- by the modalities of the information it considerable variability in the anatomic dence from functional neuroimaging has processes. findings that link the ATL with semantic not clearly pointed to the ATL as a critical In their study, subjects viewed images processing. The inconsistent findings across region for conceptual knowledge. -

Seed MNI Coordinates Lobe
MNI Coordinates Seed Lobe (Hemisphere) Region BAa X Y Z FP1 -18 62 0 Frontal Lobe (L) Medial Frontal Gyrus 10 FPz 4 62 0 Frontal Lobe (R) Medial Frontal Gyrus 10 FP2 24 60 0 Frontal Lobe (R) Superior Frontal Gyrus 10 AF7 -38 50 0 Frontal Lobe (L) Middle Frontal Gyrus 10 AF3 -30 50 24 Frontal Lobe (L) Superior Frontal Gyrus 9 AFz 4 58 30 Frontal Lobe (R) Medial Frontal Gyrus 9 AF4 36 48 20 Frontal Lobe (R) Middle Frontal Gyrus 10 AF8 42 46 -4 Frontal Lobe (R) Inferior Frontal Gyrus 10 F7 -48 26 -4 Frontal Lobe (L) Inferior Frontal Gyrus 47 F5 -48 28 18 Frontal Lobe (L) Inferior Frontal Gyrus 45 F3 -38 28 38 Frontal Lobe (L) Precentral Gyrus 9 F1 -20 30 50 Frontal Lobe (L) Superior Frontal Gyrus 8 Fz 2 32 54 Frontal Lobe (L) Superior Frontal Gyrus 8 F2 26 32 48 Frontal Lobe (R) Superior Frontal Gyrus 8 F4 42 30 34 Frontal Lobe (R) Precentral Gyrus 9 F6 50 28 14 Frontal Lobe (R) Middle Frontal Gyrus 46 F8 48 24 -8 Frontal Lobe (R) Inferior Frontal Gyrus 47 FT9 -50 -6 -36 Temporal Lobe (L) Inferior Temporal Gyrus 20 FT7 -54 2 -8 Temporal Lobe (L) Superior Temporal Gyrus 22 FC5 -56 4 22 Frontal Lobe (L) Precentral Gyrus 6 FC3 -44 6 48 Frontal Lobe (L) Middle Frontal Gyrus 6 FC1 -22 6 64 Frontal Lobe (L) Middle Frontal Gyrus 6 FCz 4 6 66 Frontal Lobe (R) Medial Frontal Gyrus 6 FC2 28 8 60 Frontal Lobe (R) Sub-Gyral 6 FC4 48 8 42 Frontal Lobe (R) Middle Frontal Gyrus 6 FC6 58 6 16 Frontal Lobe (R) Inferior Frontal Gyrus 44 FT8 54 2 -12 Temporal Lobe (R) Superior Temporal Gyrus 38 FT10 50 -6 -38 Temporal Lobe (R) Inferior Temporal Gyrus 20 T7/T3 -

Neural Correlates of Strategic Memory Retrieval: Differentiating Between Spatial-Associative and Temporal-Associative Strategies
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by MPG.PuRe r Human Brain Mapping 29:1068–1079 (2008) r Neural Correlates of Strategic Memory Retrieval: Differentiating Between Spatial-Associative and Temporal-Associative Strategies Mischa de Rover,1* Karl Magnus Petersson,1 Sieberen P. van der Werf,2 Alexander R. Cools,3 Hans J. Berger,2 and Guille´n Ferna´ndez1,4 1F.C. Donders Center for Cognitive Neuroimaging, Radboud University Nijmegen, 6500 HB Nijmegen, The Netherlands 2Department of Medical Psychology, University Medical Centre Nijmegen, 6500 HB Nijmegen, The Netherlands 3Department of Psychoneuropharmacology, University Medical Centre Nijmegen, 6500 HB Nijmegen, The Netherlands 4Department of Neurology, University Medical Centre Nijmegen, 6500 HB Nijmegen, The Netherlands Abstract: Remembering complex, multidimensional information typically requires strategic memory re- trieval, during which information is structured, for instance by spatial- or temporal associations. Although brain regions involved in strategic memory retrieval in general have been identified, differences in re- trieval operations related to distinct retrieval strategies are not well-understood. Thus, our aim was to identify brain regions whose activity is differentially involved in spatial-associative and temporal-associa- tive retrieval. First, we showed that our behavioral paradigm probing memory for a set of object-location associations promoted the use of a spatial-associative structure following an encoding condition -

Connections of Inferior Temporal Areas TE and TEO with Medial Temporal-Lobe Structures in Infant and Adult Monkeys
The Journal of Neuroscience, April 1991, 17(4): 1095-I 116 Connections of Inferior Temporal Areas TE and TEO with Medial Temporal-Lobe Structures in Infant and Adult Monkeys M. J. Webster, L. G. Ungerleider, and J. Bachevalier Laboratory of Neuropsychology, National Institute of Mental Health, Bethesda, Maryland 20892 As part of a long-term study designed to examine the on- tion. Both elimination and refinement of projections thus ap- togeny of visual memory in monkeys and its underlying neu- pear to characterize the maturation of axonal pathways be- ral circuitry, we have examined the connections between tween the inferior temporal cortex and medial temporal-lobe inferior temporal cortex and medial temporal-lobe structures structures in monkeys. in infant and adult monkeys. Inferior temporal cortical areas TEO and TE were injected with WGA conjugated to HRP and In the course of neural development, numerous mechanisms tritiated amino acids, respectively, or vice versa, in 1 -week- are at play to achieve the final configuration of the mature brain. old and 3-4-yr-old Macaca mulatta, and the distributions of These mechanismsinclude cell death, the growth of dendritic labeled cells and terminals were examined in both limbic spines,and the remodeling of connections. Such remodeling can structures and temporal-lobe cortical areas. In adult mon- take 2 forms. In the first instance, projections become more keys, inferior temporal-limbic connections included projec- restricted. That is, they initially terminate in the appropriate tions from area TEO to the dorsal portion of the lateral nu- area of the brain, but their target fields are considerably more cleus of the amygdala and from area TE to the lateral and widespreadin infancy than they will be later in adulthood. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
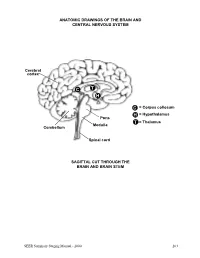
Brain and Central Nervous System
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

Neuromodulation in Epilepsy and in Chronic Pain
BlackwellMalden,NERNeuromodulation1094-715910.1111/j.1525-1403.2006.00046.x92Proceedings USA PublishingPublishing, Inc 2004 92Proceedings[squf ] Proceedings [squf ] PROCEEDINGS Neuromodulation in Epilepsy and in Chronic Pain Third Meeting of the Benelux Neuromodulation Society Chapter of the International Neuromodulation Society November 18–19, 2004, Ghent, Belgium General92Proceedings[squf ] Proceedings [squf ] Introduction to Epilepsy and Treatment the reasons of the intractability of epilepsy and the lack Modalities by Neuromodulation of success of localized resective surgery. It also empha- S. A. Chkhenkeli sizes how essential the intensive study of new methods Department of Neurology, University of Chicago, of suppression of the activity of epileptic foci is, with Chicago, Illinois, USA, and Department of Functional or without ablative surgical procedures. The concept of Neurosurgery, Epilepsy Surgery Center, the modulating epileptic focus activity and preventing or Saradzhishvili Institute of Clinical and Experimental terminating seizure activity by focal brain stimulation is Neurology, Tbilisi, Georgia attracting considerable attention. To this point, the treatment of epilepsy by electrical neuromodulation is under the causeless deep influence of the methods of Objective focal brain stimulation in movement disorders, without taking into consideration the substantial differences in Because the probability of resistant patients to achieve pathophysiological mechanisms of these diseases. complete remission with new antiepileptic drugs appears to be less than 10%, surgical interventions remain the main option to treat patients with intracta- ble epilepsy. However, results do not remain successful Materials and Methods enough, since only 29–65% of the patients were free of Studies of the organization of epileptic systems, dynam- seizures after temporal lobe and/or localized neocorti- ics of epileptic activity, and inhibitory effects of cal resections. -

Schaer K., Jahn G., Lotze M. (2012) Fmri-Activation During Drawing A
Behavioural Brain Research 233 (2012) 209–216 Contents lists available at SciVerse ScienceDirect Behavioural Brain Research j ournal homepage: www.elsevier.com/locate/bbr Research report fMRI-activation during drawing a naturalistic or sketchy portrait a b a,∗ K. Schaer ,G.Jahn , M. Lotze a Functional Imaging Unit, Center for Diagnostic Radiology and Neuroradiology, University of Greifswald, Greifswald, Germany b Department of Psychology, University of Greifswald, Greifswald, Germany h i g h l i g h t s We used fMRI to measure 20 naive subjects during drawing a portrait. Participants were able to track their drawing online. We identified three important circuits specific for the process of portrait drawing. Circuits where: face perception, location encoding, and continuous feedback processes. Representations involved: fusiform gyrus, precuneus, parietal sulcus, and cerebellum. a r t i c l e i n f o a b s t r a c t Article history: Neural processes for naturalistic drawing might be discerned into object recognition and analysis, atten- Received 8 March 2012 tion processes guiding eye hand interaction, encoding of visual features in an allocentric reference frame, Received in revised form 3 May 2012 a transfer into the motor command and precise motor guidance with tight sensorimotor feedback. Cere- Accepted 8 May 2012 bral representations in a real life paradigm during naturalistic drawing have sparsely been investigated. Available online 15 May 2012 Using a functional Magnetic Resonance Imaging (fMRI) paradigm we measured 20 naive subjects during drawing a portrait from a frontal face presented as a photograph. Participants were asked to draw the Keywords: portrait in either a naturalistic or a sketchy characteristic way.