Submission for MD
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstracts Presented at the Thirtieth Annual International Neuropsychological Society Conference
VU Research Portal The impact of the early frontal lobe damage on the development of executive functions Smidts, D.P.; Anderson, V. published in Journal of the International Neuropsychological Society 2003 document version Publisher's PDF, also known as Version of record Link to publication in VU Research Portal citation for published version (APA) Smidts, D. P., & Anderson, V. (2003). The impact of the early frontal lobe damage on the development of executive functions. Journal of the International Neuropsychological Society, 8 (2). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. E-mail address: [email protected] Download date: 03. Oct. 2021 Journal of the International Neuropsychological Society (2002), 8, 143–321. Copyright © 2002 INS. Published by Cambridge University Press. Printed in the USA. DOI: Abstracts Presented at the Thirtieth Annual International Neuropsychological Society Conference February 13–16, 2002 Toronto, Canada Wednesday Afternoon, February 13, 2002 Poster Session 1/4:00–7:00 p.m. -

Abstract Book
Plenary Abstracts PL01 Date: Friday 22 April 2016 Session: Advances Towards Prevention, Treatment and Cure LATEST DIAGNOSIS AND TREATMENT 2016 Serge Gauthier1 1McGill Center for Studies in Aging, AD Research Unit, H4H 1R3, Montreal, 6825 LaSalle Boulevard, CANADA Abstract: There are ongoing efforts at harmonizing research diagnostic criteria for Alzheimer’s disease (AD) throughout its stages (asymptomatic at risk, MCI, dementia). This will facilitate research on prevention using non-pharmacologic and pharmacologic strategies. There is a concerted effort from governments, regulators, the pharmaceutical industry and investigators to accelerate the pace of therapeutic research through all these stages of AD. Recruiting for therapeutic research will be facilitated by the involvement of persons at risk and patients through “trial ready cohorts”. The increasing use of biomarkers measuring the amount of amyloid and tau pathology in the brain may help to choose the right treatment for the right person at the right stage of disease. In the near future combinations of treatments will be tried, and we need to learn from the fields of infectious disease and cancer about trial design, practice guidelines and costs sharing. National plans will help implement current and future diagnostic and treatment strategies world-wide. Keywords: research 2 | www.adi2016.org @ADIConferenve / ADI2016 Plenary Abstracts PL02 Date: Friday 22 April 2016 Session: Advances Towards Prevention, Treatment and Cure THE WELL-EDUCATED LAB RAT: A VIEW OF CLINICAL RESEARCH FROM -

Final Program, the International Neuropsychological Society
Journal of the International Neuropsychological Society (2009 ), 1 5, Su ppl. 2. C opy right © INS. Published by Cambridge University Press, 2009. doi: 10.1017/S135561770 9 9 9 1044 Final Program The International Neuropsychological Society, Finnish Neuropsychological Society, Joint Mid-Year Meeting July 29-August 1, 2009 Helsinki, Finland & Tallinn, Estonia WEDNESDAY, JULY 29, 2009 9:00 AM–12:00 PM Continuing Education Course 1: Cerebral Palsy And Traumatic Brain Injury: A Family-Based Approach To The Rehabilitation Of The Child Presenter: Lucia Braga Press Room 9:00 AM–12:00 PM Continuing Education Course 2: Clinical & Psychometric Strategies For Improving Accuracy For Identifying Cognitive Impairment Presenter: Grant Iverson Fennia II 1:00–4:00 PM Continuing Education Course 3: Neuropsychotherapy: Guidelines For A New Integrated Field Of Neuropsychological Treatment Presenter: Ritva Laaksonen Fennia I 1:00–4:00 PM Continuing Education Course 4: The Functional Neuroanatomy Of Semantic Memory Presenter: Alex Martin Fennia II 1:00–4:00 PM Continuing Education Course 5: Neurodevelopmental Consequences Of Very Low Birth Weight: Current Knowledge And Implications Presenter: H. Gerry Taylor Press Room 4:15–4:45 PM Opening Ceremony Europaea 4:45–5:30 PM Presidential Address: Time, Language, and the Human Brain INS President: Michael Corballis Europaea 1. CORBALLIS, M Time, Language, and the Human Brain. 6:00–7:30 PM Helsinki City Reception Helsinki City Hall Downloaded from https://www.cambridge.org/core. IP address: 170.106.35.93, on 26 Sep 2021 at 02:03:36, subject to the Cambridge Core terms of use, available at https://www.cambridge.org/core/terms. -

ANNEXES COMPLEMENTAIRES Repérage Et Processus Menant Au
ANNEXES COMPLEMENTAIRES Repérage et processus menant au diagnostic de la maladie d’Alzheimer et d’autres troubles neurocognitifs Octobre 2015 Une production de l’Institut national d’excellence en santé et en services sociaux Le présent document contient les annexes complémentaires au rapport « Repérage et processus menant au diagnostic de la maladie d'Alzheimer et d'autres troubles neurocognitifs ». Ces annexes et le rapport final sont accessibles en ligne dans la section Publications de notre site Web. Le contenu de cette publication a été rédigé et édité par l’INESSS. Renseignements Institut national d’excellence en santé et en services sociaux (INESSS) 2535, boulevard Laurier, 5e étage 2021, avenue Union, bureau 10.083 Québec (Québec) G1V 4M3 Montréal (Québec) H3A 2S9 Téléphone : 418 643-1339 Téléphone : 514 873-2563 Télécopieur : 418 646-8349 Télécopieur : 514 873-1369 [email protected] www.inesss.qc.ca Responsabilité L’Institut rend accessibles les principales informations qui ont servi à la préparation du rapport « Repérage et processus menant au diagnostic de la maladie d'Alzheimer et d'autres troubles neurocognitifs » aux lecteurs qui désirent plus de détails sur sa démarche scientifique. Ce document n’a pas fait l’objet d’une révision linguistique. Il ne reflète pas forcément les opinions des autres personnes consultées aux fins du présent dossier. TABLE DES MATIÈRES SIGLES ET ACRONYMES ................................................................................................................................ i ANNEXE A -

Aspects of the Neuropsychological Development and Assessment of New Zealand Children
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Aspects of the Neuropsychological Development and Assessment of New Zealand Children A thesis presented in partial fulfilment of the requirements for the degree of Doctor of Clinical Psychology at Massey University, Wellington, New Zealand. Kate Sarah Ross-McAlpine 2018 Abstract Typical neuropsychological development in school age children is an under-researched area. There is insufficient research on age effects on performance, relationships between multiple cognitive abilities and between these abilities and academic achievement. In addition to this, there has been no research conducted on neuropsychological assessment practices with children in New Zealand (NZ). This thesis explored patterns of neuropsychological development in typically developing children and provides clarity on the current practices of psychologists conducting neuropsychological assessment with children in NZ. Study 1 explored the age effects on neuropsychological measures for typically developing children aged 6 to 11 years. Firstly, the scaled scores of NZ children were compared with overseas normative groups and found to be within ±0.4 of a standard deviation for all tests except for finger tapping and animal sorting (NEPSY-II). Secondly, age effects were found for all measures of cognitive abilities which is consistent with previous research. Post-hoc findings identified that the most significant improvement occurred between ages 6 and 9 years. The existence of differences between NZ and USA samples, specifically found for animal sorting and finger tapping (NEPSY-II), indicates that New Zealand normative data would be beneficial for some subtests used in neuropsychological assessments. -

Abstracts Presented at the Thirtieth Annual International Neuropsychological Society Conference
Journal of the International Neuropsychological Society (2002), 8, 143–321. Copyright © 2002 INS. Published by Cambridge University Press. Printed in the USA. DOI: Abstracts Presented at the Thirtieth Annual International Neuropsychological Society Conference February 13–16, 2002 Toronto, Canada Wednesday Afternoon, February 13, 2002 Poster Session 1/4:00–7:00 p.m. 3-stage odor discrimination test prior to participation. Multivariate analy- ses revealed a decline in recognition accuracy with age across all 3 do- mains, with less impairment in figural memory among individuals older AGING than 60. Moreover, age-related changes in odor memory were not related to changes in verbal or figural recognition. On the figural recognition task, T. KOGURE & T. HATTA. Temporal Memory Illusion of Social Events older individuals demonstrated more liberal response biases than did youn- in Older and Younger Adults. ger participants. Age-related differences in recognition discrimination and Even though people could remember precise contents of a social event response bias were not accounted for by global cognitive function, smok- (e.g., news event) that happened previously, the time of the event occur- ing history, sex, or ethnicity. These findings suggest that odor recognition rence could hardly be retrieved accurately. People sometimes estimate the declines with age, even among individuals with generally intact olfactory time of occurrence more recently than the actual time (telescoping bias), acuity. while they sometimes, in contrast, estimate the time of occurrence more Correspondence: Claire McGrath, Brain Behavior Laboratory, Neuropsy- remotely than the actual time (time expansion bias). The present study chiatry Section, University of Pennsylvania, Department of Psychiatry, examined aging effects of these temporal memory illusions. -

Cognitive Dysfunction in Sleep-Related Breathing Disorders: a Meta-Analysis
Sleep Research Online 5(1): 13-43, 2003 1096-214X http://www.sro.org/2003/Fulda/13/ © 2003 WebSciences Printed in the USA. All rights reserved. Cognitive Dysfunction in Sleep-related Breathing Disorders: A Meta-analysis Stephany Fulda* and Hartmut Schulz** *Krankenhaus der Barmherzigen Brüder, München, Germany **Department of Neurology, Helios Klinikum Erfurt, Erfurt, Germany Sleep related breathing disorders (SRBD) are usually associated with impaired daytime functioning. The magnitude of this impairment might vary for different neuropsychological functions. Our objective was to assess cognitive dysfunction in SRBD patients. Different medical and psychological databases (Evidence Based Medicine, Medline, Embase, PsychInfo, PsychLit, The Eric Database, BiblioSleep) were searched (last search, December 2000). The reference lists of articles were checked and several journals and conference proceedings were hand-searched. All observational studies comparing patients with an established diagnosis of SRBD to non-sleep disordered control groups, to clinical control groups, or to population norms on neuropsychological or psychometric performance measures, including computer-assisted tests and driving simulators. We rated the quality of each study according to criteria of external validity, internal validity, statistical validity, and the level of evidence. Outcome measures were classified according to a taxonomy of neuropsychological functions and statistically analyzed using meta-analytical techniques. Fifty-four studies reporting cognitive functioning of SRBD patients were reviewed. A total of 1,635 patients were compared with 1,737 control subjects. Twenty-eight studies provided adequate statistics and were integrated further. SRBD patients showed moderate to large reductions in mental flexibility, visual delayed- memory retrieval, and driving simulation performance (pooled effect size estimates ranged from 0.61 to 0.72). -

A New Software for Rehabilitation Using Touch-Screen Technology
Sede Amministrativa: Università degli Studi di Padova Dipartimento di Psicologia Generale CORSO DI DOTTORATO DI RICERCA IN SCIENZE PSICOLOGICHE CICLO XXIX New frontiers in neuropsychology. The Padua Rehabilitation Tool: a new software for rehabilitation using touch-screen technology Coordinatore: Ch.mo Prof. Francesca Peressotti Supervisore: Ch.mo Prof. Daniela Mapelli Co-Supervisore: Ch.mo Prof. Luciano Gamberini Dottorando: Stefano Cardullo Summary Recently, the advancement and the development of new technologies is shaping and establishing new frontiers in neuropsychological rehabilitation. In particular, the use of touchscreen technology, together with the use of mobile devices, is giving new opportunities for the development of innovative programs of rehabilitation tailored to the specific needs of patients. The touchscreen technology allows to go beyond some limits of classic paper and pencil exercise including the possibility for patients of practice rehabilitation outside clinic in a personalized manner and controlled by remote by the clinician. The overview of software for rehabilitation today is wide, but the poor availability of such software for Italian population and specifically designed for people with cognitive impairments, some years ago, led me to the development of the first mobile devices’ software for cognitive rehabilitation. The aim of this dissertation is to describe the development process and the efficacy evaluation of this software, the Padua Rehabilitation Tool (PRT). Initially will be described and analyzed the base for every cognitive intervention: the plasticity of brain. Today, we know that the brain has the ability to undergo functional and structural alterations in response to internal and external environmental changes, including cognitive interventions. Moreover, I will discuss the use of technology and computerized training for rehabilitation. -

Supporting Independence and Function in People Living with Dementia a Technical Guide to the Evidence Supporting Reablement Interventions
Supporting independence and function in people living with dementia A technical guide to the evidence supporting reablement interventions. Second Edition © 2019, 2018 the authors. You may copy, distribute, display, download or otherwise freely deal with this work for any purpose, provided that you attribute the authors as the owner. However, you must obtain permission if you wish to (a) include the work in advertising or a product sale, or (b) modify the work. Suggested citation: O’Connor CM, Poulos CJ, Gresham M, Poulos RG. Supporting independence and function in people living with dementia, A technical guide to the evidence supporting reablement interventions (2nd Edition). Sydney: HammondCare, 2019. ISBN 978-0-6483387-4-1 Cover design: Melissa Summers of SD Creative Funding and acknowledgements This project was funded by the National Health and Medical Research Council (NHMRC) Partnership Centre for Dealing with Cognitive and Related Functional Decline in Older People. The opinions reflected in this document do not necessarily reflect the views of the Funding Partners of the Partnership Centre. The project was a partnership between HammondCare, University of New South Wales, Brightwater Care Group, Helping Hand Aged Care, Dementia Australia, and the Department of Health. The team comprised a range of researchers, clinicians, policy makers, aged care providers, and also people impacted by dementia, both people living with dementia and the people supporting them. Collaboration with consumers has been an important element of the process, -

2015 Sydney Program Book
CONFERENCE PROGRAM 1-4 July 2015 Sofitel Sydney Wentworth 1 WELCOME TO DELEGATES Dear Colleagues, The full programme with abstracts is also available to Welcome to the 5th Pacific Rim download from the ASSBI website. Conference which is incorporating the I would like to thank the Program Committee for all International Neuropsychological Society their help in putting together this very impressive (INS)’s mid-year meeting and the programme and the INS for support in many aspects of the Australasian Society for the Study of organisation. Brain Impairment (ASSBI)’s 38th Annual I would also like to thank our Major Partners: Business Brain Impairment Conference. This will Events Sydney, Lifetime Care and Support Authority, our be the largest of the conferences borne Headline Partners Epworth Rehabilitation, Transport of the longstanding and fruitful partnership between these Accident Commission and Shine Lawyers as well as the multidisciplinary organisations. The theme of the American Psychological Association, Brain Sciences UNSW conference, Implementing Knowledge to Improve and Menzies Health Institute Queensland who have Outcomes reflects the diverse interests of our sponsored our student programme. Thanks to the ARC members, from experimental studies to clinical issues, Centre of Excellence in Cognition and its Disorders. Also our across the developmental spectrum, with emphasis on Exhibitors, MRTS, Viatour Travel, Sydney Coffee, Drake making a difference to the lives of individuals with brain Medox, Taylor & Francis, Pearson Clinical Assessment, injury. Everyday Independence and Cambridge University Press as In this book you will find the conference program, all well as our satchel insert sponsors, Psych Assessments abstracts can be found on the Smartphone App which is Australia, Rehability ABI Services and Cambridge Scholars easy to download and if you haven’t done this already go to Publishing. -
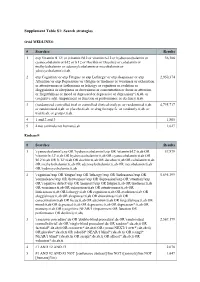
Supplement Table S1: Search Strategies
Supplement Table S1: Search strategies Ovid MEDLINE® # Searches Results 1 exp Vitamin B 12/ or (vitamin B12 or vitamin b-12 or hydroxocobalamin or 36,306 cyanocobalamin or b12 or b 12 or Docibin or Ducobee or cobalamin or methylcobalamin or adenosylcobalamin or mecobalamin or ydroxycobalamin).ti,ab. 2 exp Cognition/ or exp Fatigue/ or exp Lethargy/ or exp sleepiness/ or exp 2,950,374 Attention/ or exp Depression/ or (fatigue or tiredness or weariness or exhaustion or attentiveness or listlessness or lethargy or cognition or avolition or sluggishness or sleepiness or drowsiness or concentration or focus or attention or forgetfulness or mood or depressed or depressive or depression*).ti,ab. or (cognitive adj1 (impairment or function or performance or decline)).ti,ab. 3 (randomized controlled trial or controlled clinical trial).pt. or randomized.ti,ab. 4,795,717 or randomised.ti,ab. or placebo.ti,ab. or drug therapy.fs. or randomly.ti,ab. or trial.ti,ab. or groups.ti,ab. 4 1 and 2 and 3 1,805 5 4 not (animals not humans).sh. 1,627 Embase® # Searches Results 1 'cyanocobalamin'/exp OR 'hydroxocobalamin'/exp OR 'vitamin b12':ti,ab OR 57,879 'vitamin b-12':ti,ab OR hydroxocobalamin:ti,ab OR cyanocobalamin:ti,ab OR 'b12':ti,ab OR 'b 12':ti,ab OR docibin:ti,ab OR ducobee:ti,ab OR cobalamin:ti,ab OR methylcobalamin:ti,ab OR adenosylcobalamin:ti,ab OR mecobalamin:ti,ab OR hydroxycobalamin:ti,ab 2 'cognition'/exp OR 'fatigue'/exp OR 'lethargy'/exp OR 'listlessness'/exp OR 5,695,399 'somnolence'/exp OR 'drowsiness'/exp OR 'depression'/exp OR 'attention'/exp -

Final Program the 2012 International Neuropsychological Society Mid-Year Meeting/11Th Nordic Meeting in Neuropsychology June 27-30, 2012 Oslo, Norway
Final Program The 2012 International Neuropsychological Society Mid-Year Meeting/11th Nordic Meeting in Neuropsychology June 27-30, 2012 Oslo, Norway WEDNESDAY, JUNE 27, 2012 8:30–10:00 AM Invited Symposium: Adult ADHD — a Multifaceted Approach to Assessment and Intervention Co-Chairs: Liisa Klenberg, Laura Hokkanen København 1. HOKKANEN, L Adult ADHD – a Multifaceted Approach to Assessment and Intervention 2. LAASONEN, M Adult Dyslexia and ADHD in Finland – Project DyAdd 3. HELENIUS, P Impaired Neural Activation of the Ventral Attentional Pathway in ADHD 4. WASSERSTEIN, J Diagnosis of ADHD in Adults: Appropriate Symptom Threshold and the Role of Executive Function Measurement 5. WASSERSTEIN, J Nonpharmacological Treatment of Executive Dysfunction in Adults with ADHD 8:30–10:00 AM Paper Session: Cognitive Intervention/Rehabilitation Telemark 1. COVRE, P Reduction of Retroactive Interference may Improve Delayed Recall in Patients with Amnesia 2. ADLAM, AR Neurocognitive Interventions For Executive Function In Children Who Have Survived A Brain Injury 3. STUBBERUD, J Goal Management Training of Executive Functions in Patients with Spina Bifida 4. HERMANSEN GRUNEWALDT, K Computerized Working Memory Training in Very Low Birth Weight Children at Preschool Age 5. HASLAM, C Errorless Learning Enhances Memory in Children with Acquired Brain Injury 8:30–11:00 AM CE Workshop 1: Analysis of the Single Case in Clinical Practice: Quantitative Methods without Tears Presenter: John Crawford Oslo 1. CRAWFORD, JR Analysis of the single case in clinical practice: Quantitative methods without tears 8:30–11:00 AM CE Workshop 2: Evidence-Based Neuropsychological Assessment Presenter: Grant Iverson Helsingfors 1. IVERSON, GL Evidence-Based Neuropsychological Assessment 10:30 AM–12:00 PM Invited Symposium: Neuropsychological Studies in Autism from Denmark and Norway.