Thai Malena Mo Hinwohin Mitte
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

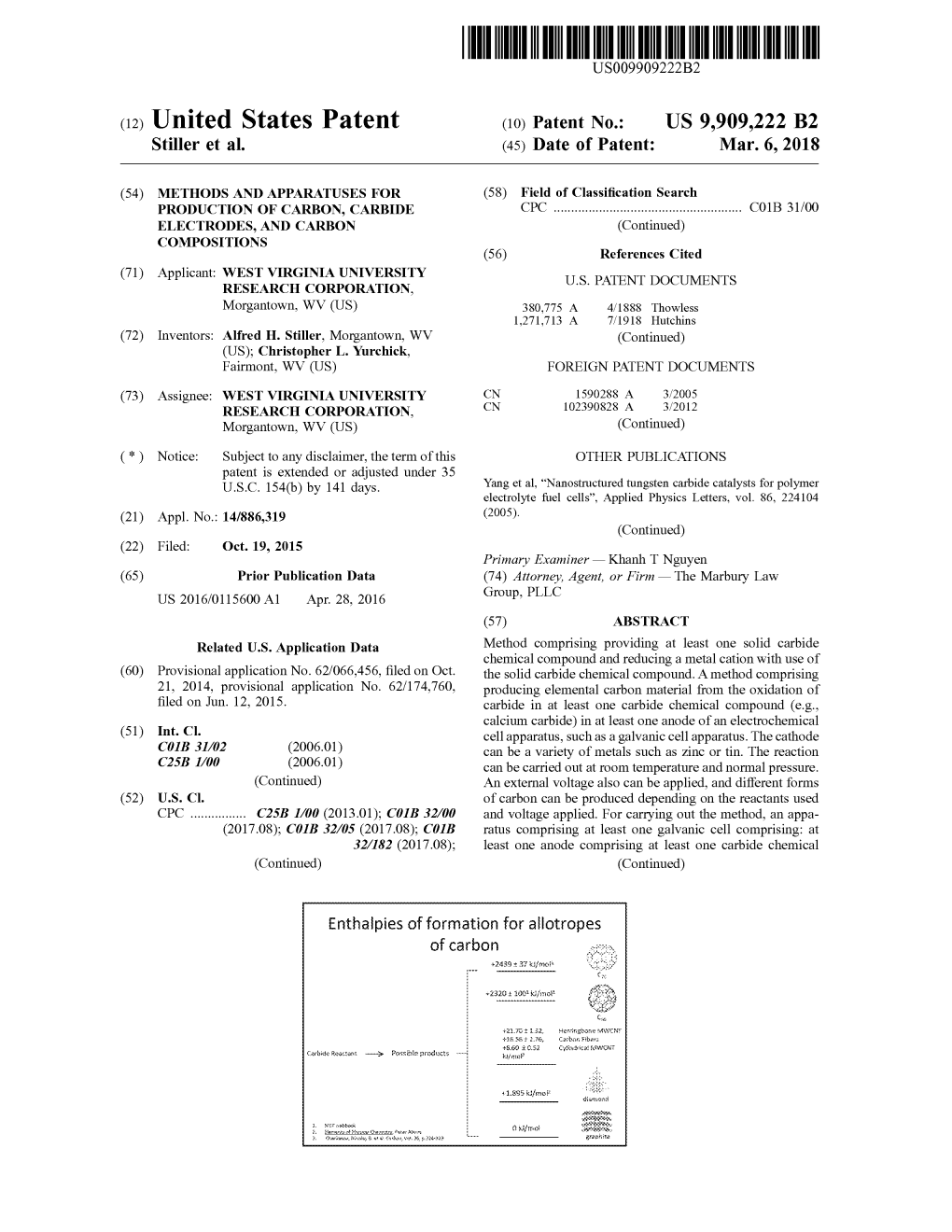
(12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub
US 20050044778A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0044778A1 Orr (43) Pub. Date: Mar. 3, 2005 (54) FUEL COMPOSITIONS EMPLOYING Publication Classification CATALYST COMBUSTION STRUCTURE (51) Int. CI.' ........ C10L 1/28; C1OL 1/24; C1OL 1/18; (76) Inventor: William C. Orr, Denver, CO (US) C1OL 1/12; C1OL 1/26 Correspondence Address: (52) U.S. Cl. ................. 44/320; 44/435; 44/378; 44/388; HOGAN & HARTSON LLP 44/385; 44/444; 44/443 ONE TABOR CENTER, SUITE 1500 1200 SEVENTEENTH ST DENVER, CO 80202 (US) (57) ABSTRACT (21) Appl. No.: 10/722,127 Metallic vapor phase fuel compositions relating to a broad (22) Filed: Nov. 24, 2003 Spectrum of pollution reducing, improved combustion per Related U.S. Application Data formance, and enhanced Stability fuel compositions for use in jet, aviation, turbine, diesel, gasoline, and other combus (63) Continuation-in-part of application No. 08/986,891, tion applications include co-combustion agents preferably filed on Dec. 8, 1997, now Pat. No. 6,652,608. including trimethoxymethylsilane. Patent Application Publication Mar. 3, 2005 US 2005/0044778A1 FIGURE 1 CALCULATING BUNSEN BURNER LAMINAR FLAME VELOCITY (LFV) OR BURNING VELOCITY (BV) CONVENTIONAL FLAME LUMINOUS FLAME Method For Calculating Bunsen Burner Laminar Flame Velocity (LHV) or Burning Velocity Requires Inside Laminar Cone Angle (0) and The Gas Velocity (Vg). LFV = A, SIN 2 x VG US 2005/0044778A1 Mar. 3, 2005 FUEL COMPOSITIONS EMPLOYING CATALYST Chart of Elements (CAS version), and mixture, wherein said COMBUSTION STRUCTURE element or derivative compound, is combustible, and option 0001) The present invention is a CIP of my U.S. -

Download Download
— Studies on Lithium Acetylide Kenneth N. Campbell and Barbara K. Campbell, The University of Notre Dame In contrast to the large amount of work done on the acetylene derivatives of sodium, potassium and calcium, little attention has been paid to the analogous compounds of lithium. In 1898 Moissani prepared lithium acetylide on a small scale, by the action of acetylene on a liquid ammonia solution of lithium. He reported that lithium acetylide was less soluble in liquid ammonia than sodium acetylide, and that when isolated from the solvent, it was less stable, undergoing decomposition with evolu- tion of acetylene. On the basis of the weight of lithium acetylide obtained from a given weight of lithium, and from the amount of acetylene liberated on hydrolysis, he assigned to lithium acetylide the formula C2Li2.C2H2.2NH3. Since that time no references to lithium acetylide or lithium alkylacetylides have appeared in the literature. It was the pur- pose of the present work, therefore, to prepare and analyze lithium acetylide and a lithium alkylacetylide, and to compare their reactions with those of the better known sodium derivatives. Experimental Procedure Preparation of Lithium and Sodium Acetylides.—Acetylene gas, washed by bubbling through concentrated sulfuric acid, was passed into two liters of liquid ammonia, while 7 g. (1 mole) of metallic lithium, cut in small pieces, was added gradually, with stirring, at a rate such that the solution did not develop a permanent deep blue color. When the solution became colorless after the addition of the last piece of lithium, the flow of acetylene was stopped. -

Safety Data Sheet
SAFETY DATA SHEET Preparation Date: 7/21/2017 Revision Date: 7/21/2017 Revision Number: G1 1. IDENTIFICATION Product identifier Product code: S1725 Product Name: SULFUR, LUMP Other means of identification Synonyms: No information available CAS #: 7704-34-9 RTECS # WS4250000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: In manufacturing sulfuric acid, carbon disulfide, sulfites, insecticides, plastics, enamels, metal-glass, cements; in vulcanizing rubber; in syntheses of dyes; in making gunpowder, matches; for bleaching wood pulp, straw, wool, silk, felt, linen; in making phosphatic fertilizers; bleaching of dried fruits; fungicide and acaricide; rodent repellent; soil conditioner; nucleating reagent for photographic film; used in cosmetics, such as acne ointments and lotions, and in antidandruff shampoos. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000. Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Flammable solids Category 2 Label elements Warning Flammable solids Product code: S1725 Product name: SULFUR, LUMP 1 / 14 Hazards not otherwise classified (HNOC) Not Applicable Other hazards Not available Precautionary Statements - Prevention Keep away from heat/sparks/open flames/hot surfaces. — No smoking Ground/bond container and receiving equipment Use explosion-proof electrical/ventilating/lighting/.../equipment Wear protective gloves Wear eye/face protection Prevent dust accumulations to minimize explosion hazard In case of fire: Use CO2, dry chemical, or foam to extinguish. -

Chemical Matrix
Chemical Matrix - Ceramics Studio Recommended Personal Protective Key Hazardous Properties Storage Location Disposal Equipment Face Body Hands Protection Respiratory Respiratory Product Form / Phase Key Composition Combustible High Toxicity High Flash Point F Flash Point Plaster Room Plaster Sanitary Drain Sanitary Compressed Gas Compressed Strong Acid/Base Strong Non-Haz Disposal Non-Haz Non-Flam Cabinet Non-Flam Glaze Mixing Room Haz Waste Disposal Waste Haz Flammables Cabinet Flammable/Explosive Potentially SensitizingPotentially MSDS Strong Oxidizer / Reducer Oxidizer Strong Cartirdge Face Shield Nitrile Gloves Nitrile Use with Local Only Exhaust Ventilation with Use Non-Flam Cupboard / Shelving / Counter Cupboard Non-Flam Chemical Splash Apron Chemical Safety Glasses Safety Chemical Flame Resistant Lab Coat Lab Resistant Flame Half Face Respitator with P100 P100 with Respitator Face Half Chemical Protective Gloves per per Gloves Protective Chemical Acetone Liquid Acetone -4 X X X X X X X Albany Slip Powder Silty glacial clay used for pottery, contains up to 30% quartz free silica according to Columbus Clay web X X X X X X MSDS Alumina Hydrate Powder Alumina Hydrate X X X X X X Angelos Copperslip Liquid (thick) Copper oxide, frit 3110, CMC gum, bentonite X X Antimony oxide Powder Antimony oxide X X X X X X X X Baking Soda Powder Sodium bicarbonate X X X X X X X Bell Dark Ball Clay Powder Kaolinite, plus up to 30% quartz free silica X X X X X X Bernard Slip Clay Powder Clay, plus up to 30% silica, some considered respirable according -

The Action of Nitrogen on Lithium Carbide. by S
‘478 GENERAL, PHYSICAL AND INORGANIC. chain action as suggested by Daneel,’ then anything which alters the degree of association of the water would influence the magnitude of this effect. In order, therefore, to estimate the degree of ionization of strong acids or alkalies in concentrated solutions, the best procedure would probably be to make use of the equation 7 = AjAb (34) in which A is the equivalent conductance of the solution for which r is desired, and above which the vapor pressure of the water is p. A’, is the equivalent conductance of fie electrolyte at infinit dilution in a solu- tion to which a suitable non-electrolyte has been added, so as to give it the same viscosity and the same vapor pressure, p, as the first solution. In the absence of any experimental data, however, equation (34) must be regarded merely as a suggestion. Summary. I. It is pointed out that in calculating the degree of ionization of an electrolyte by the conductivity method, the neglect of the viscosity effect may produce errors as high as 7 and 8 per cent., even at such low concentrations as 0.1 normal and for such simple electrolytes as uni- univalent salts. 2. The relation A = kj” (in which A is the equivalent conductance of an ion, f is the fluidity of the solution, m is a constant not far from unity and dependent chiefly upon the nature of the ion, and k is a propor- tionality constant) is proposed, provisionally as the most logical basis for formulating the viscosity correction in calculating the degree of ioniza- tion, y, in moderately concentrated solutions. -

Depositional Characteristics of Recent and Late Holocene Overwash Sandsheets in Coastal Embayments from Southeast Australia
Depositional characteristics of recent and late Holocene overwash sandsheets in coastal embayments from southeast Australia. Adam D Switzer BSc (Hons) A thesis submitted in part fulfilment of the requirements of the Doctor of Philosophy in the School of Earth and Environmental Sciences University of Wollongong February 2005 CERTIFICATION I, Adam D. Switzer, declare that this thesis, submitted in partial fulfilment of the requirements for the award of Doctor of Philosophy, in the School of earth and Environmental Sciences, University of Wollongong, is wholly my own work unless otherwise referenced or acknowledged. The document has not been submitted for qualifications at any other academic institution. Adam D. Switzer 16 February 2005 ii Acknowledgments This project has benefited considerably from the leadership and input of two very unique and different individuals, my academic supervisors Brian Jones and Ted Bryant. Working with them has provided an inspirational, fascinating and sometimes frustrating insight into the world of academic studies. This project also benefited considerably from the academic input of Charlie Bristow, Simon Haslett, Colin Woodroffe, Allan Chivas and Bryan Chenhall. A major part of the funding for this project comes from Dunmore Sand and Soil and I would sincerely like to thank Kerry Steggles, Managing Director for his support of the project. The other major funding component for the project came from an Australian Postgraduate Award (Industry) and I am greatly appreciative of that. Like many people who undertake a major project I have often needed to draw on the support of my family and friends. Naomi Riggs is the one person who heads up this team. -

Late Pleistocene Raised Coral Reefs in the Eastern Red Sea – Rabigh, Saudi Arabia Ammar Manaa University of Wollongong
University of Wollongong Research Online University of Wollongong Thesis Collection University of Wollongong Thesis Collections 2011 Late Pleistocene raised coral reefs in the eastern red sea – Rabigh, Saudi Arabia Ammar Manaa University of Wollongong Recommended Citation Manaa, Ammar, Late Pleistocene raised coral reefs in the eastern red sea – Rabigh, Saudi Arabia, Master of Science - Research thesis, School of Earth and Environmental Sciences, University of Wollongong, 2011. http://ro.uow.edu.au/theses/3501 Research Online is the open access institutional repository for the University of Wollongong. For further information contact Manager Repository Services: [email protected]. Late Pleistocene raised coral reefs in the eastern Red Sea – Rabigh, Saudi Arabia *A thesis submitted in partial fulfilment of the requirements of the award of the degree MASTER OF SCIENCE (RESEARCH) From University of Wollongong By Ammar Manaa School of Earth and Environmental Sciences 2011 2 ABSTRACT The Rabigh coast (Saudi Arabia) in the study area stretches for about 12 km between Al Kharrar Lagoon in the north and Sharm Rabigh in the south. Seven prominent Pleistocene coral reef sites were investigated with terrace heights ranging from 1 to 5 m above present sea level. In addition to field descriptions, 86 samples were collected from these seven sites to provide the data for this research. Of these seven sites, 4 of the sites were front reef, and 3 were back reef. In each of the front reef sites, there was a beach rock, upper and lower reef. The elevation of the upper and lower reef in the front reef sites ranges from 0.5 m to 3.20 m above present sea level. -

Local Peralkalinity in Peraluminous Granitic Pegmatites. I. Evidence
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2021-7790. http://www.minsocam.org/ Revision 1 1 Local peralkalinity in peraluminous granitic pegmatites. I. Evidence 2 from whewellite and hydrogen carbonate in fluid inclusions 3 YONGCHAO LIU1,2, CHRISTIAN SCHMIDT2,*, AND JIANKANG LI1 4 1MNR Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources, 5 Chinese Academy of Geological Sciences, Beijing 100037, China 6 2GFZ German Research Centre for Geosciences, Telegrafenberg, 14473 Potsdam, Germany 7 *Corresponding author: [email protected] 8 9 ABSTRACT 10 Fluid inclusions in pegmatite minerals were studied using Raman spectroscopy to 11 determine the carbon species. Carbon dioxide is very abundant in the aqueous liquid and vapor − 12 phases. Occasionally, CH4 was found in the vapor. In the aqueous liquid, HCO3 was detected in 13 fluid inclusions in tantalite-(Mn) from the Morrua Mine and in late-stage quartz from the Muiâne 14 pegmatite and the Naipa Mine, all in the Alto Ligonha District, Mozambique. Moreover, we 15 observed a carbonate (calcite group) in fluid inclusions in garnet from the Naipa Mine and in 16 beryl from the Morrua Mine, both in the Alto Ligonha District, Mozambique, and a calcite-group 17 carbonate and whewellite [CaC2O4∙H2O] in fluid inclusions in topaz from Khoroshiv, Ukraine. 18 The occurrence of oxalate is interpreted to be due to a reaction of some form of carbon (possibly 19 CO or bitumen) with a peralkaline fluid. -

Tompbnents of Fission and 'Natural Radioactivity Found in Water Are
DOCUMENT RESUME,, A ED '170 1110 SE027 bill. 0 AUTHOR Thatcher, L.L. ;And Others 1 TITLE Techniques of Water-Resources Investigations of the United. States' Geblogical.Survey. Book 5, Laboratory, Analysis. Chapter- A5, Methodscr Dete-rmination of - Radioactive Substances in Water and Fluvial Sediments. INSTITUTION -Geological Survey (Dept,: .of Interior) ,Reston, PUB DATE 77 NO Tr 95p. ;For related document, see ED 131 218 AVAILABLE FRQM Super.intendent of Documents, U.S. Government Printing Off /ce , Washington, D.C. -20402(Stock Number 024- 0.01- 02928- 6:, No price quoted) */ FDRS PRICE MF01/PC04 Plus Postage. DESCRIPTORS *ChemiCal Analysis; *Geolo'gy; *LaboratorTechniques; PhYsic; Pollution; Retource Materials; S.c ence Equipment; Water Pollution control IDENTIFIERS *RadioactiveVs- Substances; *Sediments; Water Quality ABSTkACT Analytical methods for determining important , tompbnents of fission and 'natural radioactivity found in water are reportedThe discussion of each method includes conditions for application of the method, a summary of the method, interferences, required apparatus, Procedures, calculations and estimation of precision. Isotopes considered are cesium-137, strontium-90, lead-210, radium-226. and 228, tritium, and carbon-714. `When two methods are in use for an isotope,- both methods are reported. Techniques for the collection and preservation of water samples to be anallze?d for radioactivity are also discuSsed.(CS) v. *********************************************************************** 4 Re. productions supplied -

Recent Developments in the Procedures Used at the Sscer Laboratory for the Routine Preparation of Lithium Carbide
RECENT DEVELOPMENTS IN THE PROCEDURES USED AT THE SSCER LABORATORY FOR THE ROUTINE PREPARATION OF LITHIUM CARBIDE VADIM V. SKRIPKIN and NIKOLAI N. KOVALIUKH State Scientific Centre of Environmental Radiogeochemistry, Paladina 34, Kiev, 252680 Ukraine ABSTRACT. In this paper we describe and discuss the advantages from improvements in equipment design and operating procedures developed at the State Scientific Centre of Environmental Radiogeochemistry (SSCER) laboratory in Kiev. Two experimental areas are considered, viz. 1. The direct chemisorption into a lithium alloy of carbonaceous gases produced by the controlled thermal degradation (pyrolysis) of organic materials under vacuum. This approach offers the advantage of a single stage, highly efficient and eco- nomical procedure for the production of lithium carbide. It is applicable for most types of sample material encountered in rou- tine dating work and including organic detritus dispersed in a highly (up to 95% by weight) mineral matrix and/or carbonates. Bone collagen can also be processed without the need for its prior extraction and purification. 2. A conical thin walled reaction vessel for achieving improved and reproducible recoveries in the production of lithium car- bide from CO2 gas. This apparatus allows a much improved control over the surface dependent reaction in instances where there is no option other than the direct interaction of molten lithium with prepared CO2 gas. INTRODUCTION The measurement of natural radiocarbon concentrations by liquid scintillation counting (LSC) invariably depends on the production of lithium carbide as an intermediate in benzene synthesis (Barker 1953; Polach and Stipp 1967). In theory, the chemical reaction proceeds according to 2 CO2 + 10 Li - Li2C2 + 4 LiO . -
![Arxiv:1605.07142V1 [Cond-Mat.Mtrl-Sci] 23 May 2016 Cls Ecas Edl Eopsso Ihl-Otn Li Li-Content High on Decomposes Readily Also Permi LEDC to Low Sufficiently Scales](https://docslib.b-cdn.net/cover/0746/arxiv-1605-07142v1-cond-mat-mtrl-sci-23-may-2016-cls-ecas-edl-eopsso-ihl-otn-li-li-content-high-on-decomposes-readily-also-permi-ledc-to-low-su-ciently-scales-3450746.webp)
Arxiv:1605.07142V1 [Cond-Mat.Mtrl-Sci] 23 May 2016 Cls Ecas Edl Eopsso Ihl-Otn Li Li-Content High on Decomposes Readily Also Permi LEDC to Low Sufficiently Scales
Stability of Solid Electrolyte Interphase Components on Lithium Metal and Reactive Anode Material Surfaces Kevin Leung,1∗ Fernando Soto,2 Kie Hankins,2 Perla B. Balbuena,2 and Katharine L. Harrison1 1Sandia National Laboratories, MS 1415, Albuquerque, NM 87185 2Department of Chemical Engineering, Texas A&M University, College Station, TX 77843 ∗[email protected] (Dated: May 24, 2016) Abstract Lithium ion batteries (LIB) can feature reactive anodes that operate at low potentials, such as lithium metal or silicon, passivated by solid electrolyte interphase (SEI) films. SEI is known to evolve over time as cycling proceeds. In this modeling work, we focus on the stability of two main SEI components, lithium carbonate (Li2CO3) and lithium ethylene dicarbonate (LEDC). Both components are electrochemically stable but thermodynamically unstable near the equilibrium Li+/Li(s) potential. Interfacial reactions represent one way to trigger the intrinsic thermodynamic instability. Both Li2CO3 and LEDC are predicted to exhibit exothermic reactions on lithium metal surfaces, and the barriers are sufficiently low to permit reactions on battery operation time scales. LEDC also readily decomposes on high Li-content LixSi surfaces. Our studies suggest that the innermost SEI layer on lithium metal surfaces should be a thin layer of Li2O – the arXiv:1605.07142v1 [cond-mat.mtrl-sci] 23 May 2016 only thermodynamically and kinetically stable component (in the absence of a fluoride source). This work should also be relevant to inadvertant lithium plating during battery cycling, and SEI evolution on LixSi surfaces. 1 I. INTRODUCTION Solid-electrolyte interphase (SEI) films that passivate low voltage anode surfaces are critical for lithium ion battery operations.1–6 These films arise from electrochemical reduction and subsequent breakdown of the organic solvent-based electrolyte and additive molecules, which are unstable under battery charging potentials. -

Spodumene: the Lithium Market, Resources and Processes
minerals Review Spodumene: The Lithium Market, Resources and Processes Colin Dessemond 1, Francis Lajoie-Leroux 1, Gervais Soucy 1,* , Nicolas Laroche 2 and Jean-François Magnan 2 1 Département de génie chimique et de génie biotechnologique, Université de Sherbrooke, Sherbrooke, QC J1K 2R1, Canada; [email protected] (C.D.); [email protected] (F.L.-L.) 2 Nemaska Lithium Inc., Quebec, QC G1K 3X2, Canada; [email protected] (N.L.); [email protected] (J.-F.M.) * Correspondence: [email protected]; Tel.: +1-819-821-8000 (ext: 62167) Received: 17 April 2019; Accepted: 24 May 2019; Published: 29 May 2019 Abstract: This literature review gives an overview of the lithium industry, including the lithium market, global resources, and processes of lithium compounds production. It focuses on the production of lithium compounds from spodumene minerals. Spodumene is one of the most critical minerals nowadays, due to its high lithium content and high rate of extraction. Lithium is one of the most sought-after metals, due to the ever-growing demand for lithium-ion batteries (LiBs). The data on lithium extraction from minerals is scattered through years of patents, journal articles, and proceedings; hence, requiring an in-depth review, including the comprehension of the spodumene phase system, the phase conversion processes, and the lithium extraction processes. Keywords: lithium review; spodumene processes; thermodynamic of spodumene; lithium extraction 1. Introduction Lithium is the third element of the periodic table. It is the lightest of all solid elements (d = 0.53 g cm 3 at 20 C), has the highest specific heat capacity, the smallest ionic radius of all · − ◦ the alkali metals, and a high electrochemical potential [1].