Specific Features of 5S Rrna Structure – Its Interactions with Macromolecules and Possible Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Haloarcula Quadrata Sp. Nov., a Square, Motile Archaeon Isolated from a Brine Pool in Sinai (Egypt)
International Journal of Systematic Bacteriology (1999), 49, 1 149-1 155 Printed in Great Britain Haloarcula quadrata sp. nov., a square, motile archaeon isolated from a brine pool in Sinai (Egypt) Aharon Oren,’ Antonio Ventosa,2 M. Carmen Gutierrez* and Masahiro Kamekura3 Author for correspondence: Aharon Oren. Tel: +972 2 6584951. Fax: +972 2 6528008. e-mail : orena @ shum.cc. huji. ac.il 1 Division of Microbial and The motile, predominantly square-shaped, red archaeon strain 80103O/lT, Molecular Ecology, isolated from a brine pool in the Sinai peninsula (Egypt), was characterized Institute of Life Sciences and the Moshe Shilo taxonomically. On the basis of its polar lipid composition, the nucleotide Center for Marine sequences of its two 16s rRNA genes, the DNA G+C content (60-1 molo/o) and its Biogeochemistry, The growth characteristics, the isolate could be assigned to the genus Haloarcula. Hebrew University of Jerusalem, Jerusalem However, phylogenetic analysis of the two 165 rRNA genes detected in this 91904, Israel organism and low DNA-DNA hybridization values with related Haloarcula 2 Department of species showed that strain 801030/ITis sufficiently different from the Microbiology and recognized Haloarcula species to warrant its designation as a new species. A Parasitology, Faculty of new species, Haloarcula quadrata, is therefore proposed, with strain 801030/IT Pharmacy, University of SeviIIe, SeviIIe 41012, Spain (= DSM 119273 as the type strain. 3 Noda Institute for Scientific Research, 399 Noda, Noda-shi, Chiba-ken Keywords : Haloarcula quadrata, square bacteria, archaea, halophile 278-0037, Japan INTRODUCTION this type of bacterium from a Spanish saltern was published by Torrella (1986). -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Seasonal Fluctuations in Ionic Concentrations Drive Microbial Succession in a Hypersaline Lake Community
The ISME Journal (2014) 8, 979–990 & 2014 International Society for Microbial Ecology All rights reserved 1751-7362/14 www.nature.com/ismej ORIGINAL ARTICLE Seasonal fluctuations in ionic concentrations drive microbial succession in a hypersaline lake community Sheila Podell1, Joanne B Emerson2,3, Claudia M Jones2, Juan A Ugalde1, Sue Welch4, Karla B Heidelberg5, Jillian F Banfield2,6 and Eric E Allen1,7 1Marine Biology Research Division, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA, USA; 2Department of Earth and Planetary Sciences, University of California, Berkeley, CA, USA; 3Cooperative Institute for Research in Environmental Sciences, University of Colorado, Boulder, CO, USA; 4School of Earth Sciences, Byrd Polar Research Center, Ohio State University, Columbus, OH, USA; 5Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA; 6Department of Environmental Science, Policy, and Management, University of California, Berkeley, CA, USA and 7Division of Biological Sciences, University of California, San Diego, La Jolla, CA, USA Microbial community succession was examined over a two-year period using spatially and temporally coordinated water chemistry measurements, metagenomic sequencing, phylogenetic binning and de novo metagenomic assembly in the extreme hypersaline habitat of Lake Tyrrell, Victoria, Australia. Relative abundances of Haloquadratum-related sequences were positively correlated with co-varying concentrations of potassium, magnesium and sulfate, -

The Role of Stress Proteins in Haloarchaea and Their Adaptive Response to Environmental Shifts
biomolecules Review The Role of Stress Proteins in Haloarchaea and Their Adaptive Response to Environmental Shifts Laura Matarredona ,Mónica Camacho, Basilio Zafrilla , María-José Bonete and Julia Esclapez * Agrochemistry and Biochemistry Department, Biochemistry and Molecular Biology Area, Faculty of Science, University of Alicante, Ap 99, 03080 Alicante, Spain; [email protected] (L.M.); [email protected] (M.C.); [email protected] (B.Z.); [email protected] (M.-J.B.) * Correspondence: [email protected]; Tel.: +34-965-903-880 Received: 31 July 2020; Accepted: 24 September 2020; Published: 29 September 2020 Abstract: Over the years, in order to survive in their natural environment, microbial communities have acquired adaptations to nonoptimal growth conditions. These shifts are usually related to stress conditions such as low/high solar radiation, extreme temperatures, oxidative stress, pH variations, changes in salinity, or a high concentration of heavy metals. In addition, climate change is resulting in these stress conditions becoming more significant due to the frequency and intensity of extreme weather events. The most relevant damaging effect of these stressors is protein denaturation. To cope with this effect, organisms have developed different mechanisms, wherein the stress genes play an important role in deciding which of them survive. Each organism has different responses that involve the activation of many genes and molecules as well as downregulation of other genes and pathways. Focused on salinity stress, the archaeal domain encompasses the most significant extremophiles living in high-salinity environments. To have the capacity to withstand this high salinity without losing protein structure and function, the microorganisms have distinct adaptations. -

Organization and Evolution of 5S Ribosomal Dna in the Fish Genome
In: Focus on Genome Research ISSN 1-59033-960-6 Editor: Clyde R. Williams, pp335-363 ©2004 Nova Science Publishers, Inc. Chapter X ORGANIZATION AND EVOLUTION OF 5S RIBOSOMAL DNA IN THE FISH GENOME Cesar Martins 1 and Adriane Pinto Wasko 2 Departamento de Morfologia, Instituto de Biociências, Universidade Estadual Paulista, CEP 18618-000, Botucatu, SP, Brazil. Phone/Fax +55 14 38116264, 1e-mail [email protected]; 2e-mail: [email protected] ABSTRACT In higher eukaryotes, the 5S ribosomal multigene family (5S rDNA) is tandemly organized in repeat units composed of a coding region (5S rRNA gene) and a non-transcribed spacer sequence (NTS). Although the 5S rDNA organization has been investigated in several vertebrate species, present data are concentrated in mammals and amphibians, whereas other groups, such as fishes, have been poorly studied. To further the understanding on the dynamics and evolution of 5S rDNA arrays in the vertebrate genome, recent studies have focused on the genome organization of these sequences in fish species, which represent the base group of vertebrate evolution. It was evidenced that the chromosome distribution of the 5S rDNA is quite conserved among related fish species occupying an interstitial position in the chromosomes. Although the 5S rDNA clusters have been maintained conserved in the chromosomes, changes in the nucleotide sequences and organization of the repeat units have occurred in fish species, as demonstrated by the presence of 5S rDNA variant types within and between genomes clustered in distinct chromosome environments. These variants are distributed in two major classes, suggesting that such pattern could represent a primitive condition for the fish genome, as well as for vertebrates. -

Draft Genome of Haloarcula Rubripromontorii Strain SL3, a Novel Halophilic Archaeon Isolated from the Solar Salterns of Cabo Rojo, Puerto Rico
UC Davis UC Davis Previously Published Works Title Draft genome of Haloarcula rubripromontorii strain SL3, a novel halophilic archaeon isolated from the solar salterns of Cabo Rojo, Puerto Rico. Permalink https://escholarship.org/uc/item/61m6b8d8 Authors Sánchez-Nieves, Rubén Facciotti, Marc Saavedra-Collado, Sofía et al. Publication Date 2016-03-01 DOI 10.1016/j.gdata.2016.02.005 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Genomics Data 7 (2016) 287–289 Contents lists available at ScienceDirect Genomics Data journal homepage: www.elsevier.com/locate/gdata Data in Brief Draft genome of Haloarcula rubripromontorii strain SL3, a novel halophilic archaeon isolated from the solar salterns of Cabo Rojo, Puerto Rico Rubén Sánchez-Nieves a,MarcFacciottib, Sofía Saavedra-Collado a, Lizbeth Dávila-Santiago a, Roy Rodríguez-Carrero a, Rafael Montalvo-Rodríguez a,⁎ a Biology Department, University of Puerto Rico, Mayaguez, Box 9000, 00681-9000, Puerto Rico b Biomedical Engineering and Genome Center, 451 Health Sciences Drive, Davis, CA, 95618, United States article info abstract Article history: The genus Haloarcula belongs to the family Halobacteriaceae which currently has 10 valid species. Here we report Received 1 February 2016 the draft genome sequence of strain SL3, a new species within this genus, isolated from the Solar Salterns of Cabo Accepted 5 February 2016 Rojo, Puerto Rico. Genome assembly performed using NGEN Assembler resulted in 18 contigs (N50 = Available online 6 February 2016 601,911 bp), the largest of which contains 1,023,775 bp. The genome consists of 3.97 MB and has a GC content of 61.97%. -
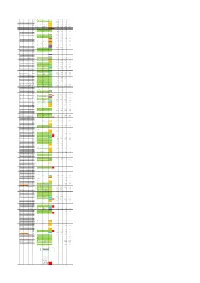
End Strand of Gene Gene Name Gene Function Starnd of Trascript
Genomic TSS strand Gene starnd of Transcipt relatively to TSS Start End Gene name coordinates relatively TSS type Control_fwd TEX_fwd Control_rev TEX_rev of gene function trascript gene group of TSS to ATG ribosomal 93 854 + rps12 + 1 protein S12 1 1 14.0638317 20.7539794 0 0 93 324 exon 1 + 111 rps12 intron 1 1 13.90756687 18.07149224 0.781323982 0.423550599 829 854 exon 2 + 496 rps12 exon2 2 24.22104343 15.24782157 0 0 30S 904 1371 + gene rps7 ribosomal + 2 protein S7 1303 -209 rps7 inter (ndhB) 2 20.47068832 9.035746118 0.625059185 0.847101199 NADH dehydrogen 1512 3535 + gene ndhB (ndh2) + ase subunit 3 2 1696 ndhB exon 1-inter 2 3.594090315 2.964854195 0.468794389 0.282367066 + 2209 ndhB exon 1-inter 2 46.09811492 4.09432246 0.468794389 0.423550599 1512 2237 exon 1 + 2756 ndhB exon 2 -inter 2 43.28534858 4.800240125 0.312529593 0.282367066 2756 3535 exon 2 + 3090 ndhB exon 2 -inter 2 17.50165719 15.95373924 0.312529593 0 + 3192 ndhB exon 2 -inter 2 140.6383167 117.6058831 2.812766334 1.694202397 - 3462 ndhB exon 2 -inter 3 1.406383167 1.129468265 1.406383167 3.67077186 4 3633 3712 + tRNA tRNA-Leu-CAA + 3610 -23 tRNA-Leu 1 77.19480938 84.85130339 0.625059185 0 + 3633 1 tRNA-Leu 1 359.5652963 649.0207016 0.781323982 0 photosyste 3954 4058 + gene psbM m II protein + 5 M 3775 -179 psbM 2 20.47068832 12.00060031 0 0.141183533 + 3954 psbM-0 2 69.22530477 28.37789015 0.156264796 0 hypothetical 4182 5073 + gene ycf66 6 protein 4182 4287 exon 1 4772 5073 exon 2 7 5202 5113 - gene ycf (ORF29) - 5299 orf29 inter 1 0 0 3.125295926 3.67077186 -

Haloarcula Marismortui (Volcani) Sp
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, Apr., 1990, p. 209-210 Vol. 40. No. 2 0020-7713/90/020209-02$02.00/0 Copyright 0 1990, International Union of Microbiological Societies Haloarcula marismortui (Volcani) sp. nov. nom. rev. an Extremely Halophilic Bacterium from the Dead Sea A. OREN,l* M. GINZBURG,2 B. Z. GINZBURG,2 L. I. HOCHSTEIN,3 AND B. E. VOLCAN14 Division of Microbial and Molecular Ecology,’ and Plant Biophysical Laboratory,2 Institute of Life Sciences, The Hebrew University of Jerusalem, 91 904 Jerusalem, Israel; National Aeronautics and Space Administration Ames Research Center, Mofett Field, California 9403j3; and Scripps Institution of Oceanography, University of California, Sun Diego, La Jolla, California 920934 An extremely halophilic red archaebacterium isolated from the Dead Sea (Ginzburg et a]., J. Gen. Physiol. 55: 187-207,1970) belongs to the genus Haloarcula and differs sufficiently from the previously described species of the genus to be designated a new species; we propose the name Haloarcula marismortui (Volcani) sp. nov., nom. rev. because of the close resemblance of this organism to “Halobacterium marismortui,” which was first described by Volcani in 1940. The type strain is strain ATCC 43049. During his studies on the microbiology of the Dead Sea in served after electrophoresis of digests of DNA preparations the 1930s and 1940s Elazari-Volcani isolated a novel strain of with different restriction enzymes (ll), and although the the genus Halobacterium. This strain differed from the then DNA-DNA hybridization ratio of these organisms is rather known halobacterial types in its ability to form acid from low, the new isolate and strain ATCC 29715 appeared to be glucose, fructose, mannose, and glycerol and in its produc- related, as shown by the near identity of their 5s and 16s tion of gas from nitrate. -

HUMAN RIBOSOME BIOGENESIS and the REGULATION of the TUMOUR SUPPRESSOR P53
HUMAN RIBOSOME BIOGENESIS AND THE REGULATION OF THE TUMOUR SUPPRESSOR p53 Andria Pelava Submitted for Doctor of Philosophy Final submission: December 2016 Institute of Cell and Molecular Biosciences Faculty of Medical Sciences Newcastle University ii Abstract Ribosome production is an energetically expensive and, therefore, highly regulated process. Defects in ribosome biogenesis lead to genetic diseases called Ribosomopathies, such as Dyskeratosis Congenita (DC), and mutations in ribosomal proteins and ribosome biogenesis factors are linked to multiple types of cancer. During ribosome biogenesis, the ribosomal RNAs (rRNAs) are processed and modified, and defects in ribosome biogenesis lead to the activation of p53. This project aimed to investigate the functions of Dyskerin, mutated in X-linked DC, in human ribosome biogenesis and p53 regulation, and to explore the link between ribosome production and p53 homeostasis. Dyskerin is an rRNA pseudouridine synthase and required for telomere maintenance. There is some debate as to whether DC is the result of telomere maintenance or ribosome biogenesis defects. It is shown here that human Dyskerin is required for the production of both LSU and SSU, and knockdown of Dyskerin leads to p53 activation via inhibition of MDM2 via the 5S RNP, an LSU assembly intermediate which accumulates after ribosome biogenesis defects. My data indicate that p53 activation, due to defects in ribosome biogenesis, may contribute to the clinical symptoms seen in patients suffering with DC. In addition, it is shown that defects in early or late stages of SSU or LSU biogenesis, result in activation of p53 via the 5S RNP-MDM2 pathway, and that p53 is induced in less than 12 hours after ribosome biogenesis defects. -

Selenoprotein H Is an Essential Regulator of Redox Homeostasis That
Selenoprotein H is an essential regulator of redox PNAS PLUS homeostasis that cooperates with p53 in development and tumorigenesis Andrew G. Coxa, Allison Tsomidesa, Andrew J. Kima, Diane Saundersa, Katie L. Hwanga, Kimberley J. Evasonb, Jerry Heidelc, Kristin K. Brownd, Min Yuand, Evan C. Liend, Byung Cheon Leea,e, Sahar Nissima, Bryan Dickinsonf, Sagar Chhangawalag, Christopher J. Changh,i, John M. Asarad, Yariv Houvrasg, Vadim N. Gladysheva,j, and Wolfram Goesslinga,j,k,l,1 aBrigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115; bUniversity of Utah, Salt Lake City, UT 84112; cOregon State University, Corvallis, OR 97331; dBeth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115; eKorea University, 02841 Seoul, Republic of Korea; fUniversity of Chicago, Chicago, IL 60637; gWeill Cornell Medical College and New York Presbyterian Hospital, New York, NY 10065; hHoward Hughes Medical Institute, Bethesda, MD 20815; iUniversity of California, Berkeley, CA 20815; jBroad Institute of MIT and Harvard, Cambridge, MA 02142; kHarvard Stem Cell Institute, Cambridge, MA 02138; and lDana-Farber Cancer Institute, Harvard Medical School, Boston, MA 02115 Edited by Leonard I. Zon, Howard Hughes Medical Institute, Boston Children’s Hospital, Harvard Medical School, Boston, MA, and accepted by Editorial Board Member Carol Prives July 18, 2016 (received for review January 7, 2016) Selenium, an essential micronutrient known for its cancer preven- mice exhibit impaired selenium transport from the liver to pe- tion properties, is incorporated into a class of selenocysteine- ripheral tissues and show growth retardation and impaired motor containing proteins (selenoproteins). Selenoprotein H (SepH) is a coordination (13, 14). -

UC Riverside Electronic Theses and Dissertations
UC Riverside UC Riverside Electronic Theses and Dissertations Title Characterization of AtRAP Function in Plant Immunity and in RNA Transportation Permalink https://escholarship.org/uc/item/8pk9k169 Author Wang, Huan Publication Date 2017 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA RIVERSIDE Characterization of AtRAP Function in Plant Immunity and in RNA Transportation A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Plant Pathology by Huan Wang December 2017 Dissertation Committee: Dr. Hailing Jin, Chairperson Dr. Julia Bailey-Serres Dr. Isgouhi Kaloshian Copyright by Huan Wang 2017 The Dissertation of Huan Wang is approved: Committee Chairperson University of California, Riverside ACKNOWLEDGEMENTS I would like to express my sincere gratitude and appreciation to all those who helped and encouraged me during my graduate study. My deepest gratitude goes first and foremost to Dr. Hailing Jin, my supervisor, for her patient and constant guidance and encouragement. No matter what the difficulty is, she always stands with me to give me very timely, experienced and smart advice to help me solve it quickly and efficiently. Her creative ideas, intelligent analysis, hardworking attitude and generous personality inspire and teach me a lot not only on my research but also on my philosophy of life. Secondly, I would like to express my heartfelt gratitude to my dissertation committee members, Dr. Julia Bailey-Serres and Dr. Isgouhi Kaloshian. I really appreciate your valuable suggestions and kindly help to my study. Dr. Julia Bailey-Serres continuously helps my study to be my guidance committee member, qualifying exam committee member, and dissertation committee member. -

Investigating Bacterial Ribosomal Sequence Variation in Regards to Future Structural and Antibiotic Research
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.14.448437; this version posted June 14, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Investigating bacterial ribosomal sequence variation in regards to future structural and antibiotic research. Helena B. Cooper1, Kurt L. Krause1 & Paul P. Gardner1. 1Department of Biochemistry, School of Biomedical Sciences, University of Otago. Author Note Keywords: Bacterial Ribosome, Antibiotic Resistance, Phylogeny Analysis, Genomics. We have no known conflicts of interest to disclose. Correspondence concerning this article should be addressed to: Paul P. Gardner, Department of Biochemistry, University of Otago, P. O. Box 56, Dunedin, 9054. Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.06.14.448437; this version posted June 14, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Ribosome-targeting antibiotics comprise over half of antibiotics used in medicine, but our fundamental knowledge of their binding sites is derived primarily from ribosome structures from non-pathogenic species. These include Thermus thermophilus, Deinococcus radiodurans and Haloarcula marismortui, as well as the commensal or pathogenic Escherichia coli. Advancements in electron cryomicroscopy have allowed for the determination of more ribosome structures from pathogenic bacteria, with each study highlighting species-specific differences that had not been observed in the non-pathogenic structures.