Examination of Micro-Quantity of Ball Point Inks from Documents by Thin-Layer Chromatography George R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Za³¹cznik Nr 1 Do SIWZ
Zał ącznik nr 1 do SIWZ 1 2 3 4 6 7 8 9 Cena Ilo ść/ jednost Ilo ść/ Ilo ść/ Cena Warto ść miejsce Poda kowa Warto ść jednostka miejsce miejsce Ogółem jednost netto LP. Nazwa asortymentu dostawy tek brutto brutto (kol.4 miary dostawy OR dostawy Ilo ść kowa (kol.4 x PT Nowy VAT (kol. 5 x kol.7) Kraków PT Tarnów netto kol.5) Sącz x kol. 6) 1 CLIPBOARD rozkładany A4 sztuk 10 0 2 12 2 CLIPBOARD rozkładany A5 sztuk 10 0 7 17 3 Cienkopis czarny typu Stabilo, Pentel, Pilot lub Kamet sztuk 100 120 100 320 4 Cienkopis czerwony typu Stabilo, Pentel, Pilot lub Kamet sztuk 100 120 30 250 5 Cienkopis niebieski typu Stabilo, Pentel, Pilot lub Kamet sztuk 100 120 20 240 6 Cienkopis zielony typu Stabilo, Pentel, Pilot lub Kamet sztuk 100 120 20 240 7 Cienkopis kulkowy typu 411 COOL STAEDTLER kolor niebieski sztuk 0 0 10 10 8 Datownik automatyczny typu Trodat 4810 sztuk 50 45 0 95 9 Długopis czarny zwykły typu Stabilo, Pentel lub Pilot sztuk 100 0 0 100 10 Długopis niebieski zwykły typu Stabilo, Pentel lub Pilot sztuk 100 0 0 100 11 Długopis Ŝelowy niebieski typu Stabilo, Pentel lub Pilot sztuk 100 60 0 160 12 Długopis Ŝelowy czarny typu Stabilo, Pentel lub Pilot sztuk 100 60 0 160 13 Długopis Ŝelowy czerwony typu Stabilo, Pentel lub Pilot sztuk 50 60 0 110 14 Długopis przyklejany leŜący na spręŜynce sztuk 30 130 0 160 15 Długopis typu ZENITH, Ballograf, Parker na wkłady wielkopojemne sztuk 50 160 160 370 16 Dziurkacz duŜy typu Esselte, Eagle, Raxel na 60kartek sztuk 20 0 0 20 17 Dziurkacz mały typu Esselte, Eagle, Raxel na 20kartek sztuk 50 20 10 80 18 Dziennik korespondencyjny A4 sztuk 100 120 50 270 19 Flamaster czarny cienki typu Stabilo, Pentel lub Pilot sztuk 100 150 0 250 20 Flamaster czarny gruby typu Stabilo, Pentel lub Pilot sztuk 150 0 0 150 21 Gumka do mazania typu Pentel, Pilot,Pelikan lub Rasoplsast sztuk 50 160 100 310 22 Gumka recepturka duŜa (średnica min: 15 cm) w opakowaniu 1 kg opak. -

Other Grease Pencils and Markers
OTHER GREASE PENCILS AND MARKERS After writing an online essay about Listo mechanical grease pencils (aka china markers, crayon pencils, etc.), I took an inventory of all the other brands of “markers” that had stuck to my hands over the years. I found two dozen! When I stroll the outdoor and indoor antiques and collectibles markets in this area, I pick up anything that looks like fun and is in my price range, i.e. cheap - - Frank Dubiel (RIP) and I had a lot in common. (Also, if you’ve read my other essays, you know I am first and foremost an Autopoint, Realite and Realpoint collector.) So I’m going to show you a sort of “parade” of markers which 1)vary between mechanical instruments and simple “clamp the lead” devices, 2)use a wide variety of diameters of grease or crayon type leads, and 3)are made from various materials like wood, plastic, and metal. I’ll lead off with Autopoint’s sole offering first, then retreat to what I suspect is the oldest marker in my collection, and work toward the most modern of the markers that I found. A WORD ABOUT MARKERS AND LEAD REFILLS SIZES: Autopoint, Listo and many other manufacturers made “checking pencils” along with their production of “normal” mechanical pencils, especially in the 1920s, 1930s and 1940s. Autopoint probably made a hundred different styles of “regular” mechanical pencils, but it also made a wide variety of “checking” pencils over those years. Most of Autopoint’s early mechanical pencils either used .046” diameter (“standard”) or .036” diameter (“real thin”) leads, but its “checking” leads were .076” in diameter (or ~1.93mm). -

A Review of Collectable Fountain Pens by Dave Wells
Personal Accessories A Review of Collectable Fountain Pens by Dave Wells. A Council Member of the Writing Equipment Society This feature has been timed to coincide with The London Writing Equipment Show which is sponsored by the Parket Pen Company and organised by the UK Writing Equipment Society. This will be held on Sunday 7th October at Kensington Town Hall, Hornton Street London. 11am-5pm. Entry for the public £5. Early ink-holding pens dating from the late nineteenth and beginning of twentieth centuries had screw off barrel and filling using a glass eye dropper. There are many beautiful examples which can be of high value to collectors especially when they include decoration such as moulded gold bands and ends and mother of pearl. Where the nib is exposed for writing by a screwing mechanism, the correct description is that of a Safety Pen. Fig 1 is an 18kt gold Ital Pen Co model, made in the United States around 1910. When the ink flow rate is controlled internally by a feed system to the nib then it is a true Fountain Pen. The next challenge after stabilising ink flow was to find a clean and easy way of ‘self filling’. In 1905 De La Rue developed the plunger-filling Onoto. Other solutions used a rubber ink sac. Sheaffer introduced its pioneering ‘lever filler’ and Parker had an end button filler (with button plus pressure bar to squash the sac). Figs 2 and 3 show a 1920 Waterman #456 with silver overlay and a Parker Duofold Senior from 1927. Since that time there have been many different filler methods of Fig 5. -
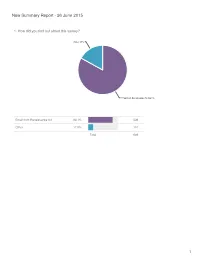
New Summary Report - 26 June 2015
New Summary Report - 26 June 2015 1. How did you find out about this survey? Other 17% Email from Renaissance Art 83.1% Email from Renaissance Art 83.1% 539 Other 17.0% 110 Total 649 1 2. Where are you from? Australia/New Zealand 3.2% Asia 3.7% Europe 7.9% North America 85.2% North America 85.2% 553 Europe 7.9% 51 Asia 3.7% 24 Australia/New Zealand 3.2% 21 Total 649 2 3. What is your age range? old fart like me 15.4% 21-30 22% 51-60 23.3% 31-40 16.8% 41-50 22.5% Statistics 21-30 22.0% 143 Sum 20,069.0 31-40 16.8% 109 Average 36.6 41-50 22.5% 146 StdDev 11.5 51-60 23.3% 151 Max 51.0 old fart like me 15.4% 100 Total 649 3 4. How many fountain pens are in your collection? 1-5 23.3% over 20 35.8% 6-10 23.9% 11-20 17.1% Statistics 1-5 23.3% 151 Sum 2,302.0 6-10 23.9% 155 Average 5.5 11-20 17.1% 111 StdDev 3.9 over 20 35.8% 232 Max 11.0 Total 649 4 5. How many pens do you usually keep inked? over 10 10.3% 7-10 12.6% 1-3 40.7% 4-6 36.4% Statistics 1-3 40.7% 264 Sum 1,782.0 4-6 36.4% 236 Average 3.1 7-10 12.6% 82 StdDev 2.1 over 10 10.3% 67 Max 7.0 Total 649 5 6. -

Catalog #70 Final Print Copy
Gary & Myrna Lehrer’s Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #70 - March 2014 See the Catalog in full color on the web site. For about a week you’ll need a password for access (be sure to also see what’s remaining from previous Catalogs). WEBSITE PAS SWORD FOR CATALOG #70: (www.gopens.com): SPRING Catalog #70 Features: Featured are both Conklin Endura and Delta LE Pens Rare Pelikans; Parker “Cloud” Series Pens Some incredible Italian and German Pens 15+ Manufacturers Represented Rare Zaner Bloser; Waterman 416 Over 250 Items Contact Information: Tel: (203) 389-5295 email: [email protected] Fax: (859) 909-1882 Call until 9:00 PM Eastern Time; Fax or email anytime We check our email often Subscription Expired: A (4) on your mailing label means your subscription has expired. “Internet Only” renewal is $10. “Hard Copy” Renewal is $25 US and $35 Foreign (see website for details). Received a sample copy? Don’t forget to subscribe ! Please see inside front page for abbreviations and other important information! Gary & Myrna Lehrer 16 Mulberry Road Woodbridge, CT 06525-1717 March 2014 - CATALOG #70 Here’s Some Other Important Information : GIFT CERTIFICATES: Available in any denomination. No extra cost! No expiration! CONSIGNMENT - PEN PURCHASES : We usually accept a small number of consignments. Ask about consignment rates (we reserve the right to turn down consignments), or see the website for details. We are also always looking to purchase one pen or entire collections. ABBREVIATIONS : Mint - No sign of use Fine - Used, parts show wear Near Mint - Slightest signs of use Good - Well used, imprints may be almost Excellent - Imprints good, writes well, looks great gone, plating wear Fine+ - One of the following: some brassing, Fair - A parts pen some darkening, or some wear ---------------------------------------------------------------------------------------------------------------------------------------- LF - Lever Filler HR - Hard Rubber PF - Plunger Filler (ie. -

Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #57 - December 2010
Gary & Myrna Lehrer’s Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #57 - December 2010 See the Catalog in full color on the web site. For about a week you’ll need a password for access (be sure to also see what’s remaining from previous Catalogs). WEB SITE PAS SWORD FOR CATALOG #57: (www.gopens.com): BOOK Catalog #57 Feature: New-old-stock, Mint-in-box items for your gift list Incredible “Extraordinary Pens” Over 265 Items 20+ Manufacturers Represented Rider “Snake” & Waterman Fine-Silver Taper Cap Pens Very rare Pelikans & several Wahl Oversize Deco-bands Contact Information: Tel: (203) 389-5295 email: [email protected] Fax: (859) 909-1882 Call until 10:30 PM Eastern Time; Fax anytime We check our email often Subscription Expired: A (4) on your mailing label means your subscription has expired. “Internet Only” renewal is $10. “Hard Copy” Renewal is $25 US and $35 Foreign (see website for details). Received a sample copy? Don’t forget to subscribe ! Please see inside front page for abbreviations and other important information! Gary & Myrna Lehrer 16 Mulberry Road Woodbridge, CT 06525-1717 December 2010 - CATALOG #57 Here’s Some Other Important Information : GIFT CERTIFICATES : Available in any denomination. No extra cost! No expiration! Always fully refundable! REPAIRS - CONSIGNMENT - PEN PURCHASES : We do the full array of pen repairs - very competitively priced. Ask about consignment rates for the Catalog (we reserve the right to turn down consignments), or see the web site for details. We are also always looking to purchase one pen or entire collections. -

CATALOGUE2020 Sve Webb.Pdf
Redan 1945 började Ballograf sin produktion i ett garage i Göteborg och idag kan vi stoltsera med att vara Sveriges enda penntillverkare. Genom åren har vi kontinuerligt utvecklat och förbättrat våra produkter, så det är med gott samvete vi lämnar livstids garanti på varje penna som lämnar vår fabrik. Vi arbetar med noggrant utvalda material för att kunna garantera att våra pen- nor håller vad vi lovar och vi har fått utmärkelser för vår genomarbetade design. Våra pennor säljs idag framgångsrikt i bland annat Japan, Schweiz, Finland, Italien, Tyskland, Österrike och USA. Idag tillverkas årligen över 5 miljoner kulspetspennor, stiftpennor och tuschpen- nor i fabriken i Filipstad. The story of Ballograf began back in 1945 in a garage in Gothenburg, and today we’re proud to say that we’re the only Swedish pen manufacturer. Through the years we’ve continuously developed and improved our products, so it is with a clear conscience we leave a lifetime guarantee on every pen that leaves our factory. We work with carefully selected materials to ensure that our pens live up to our high standars and we’ve received awards for our elaborate design. Our pens are currently sold successfully in Japan, Switzerland, Finland, Italy, Germany, Austria and the USA. To day Ballograf manufactures over 5 million ballpoint pens, mechanical pencils and markers anually at the factory in Filipstad. 1 2 EXECUTIVE Kulspetspenna Ballpoint Pen Ballograf Permo lanserades på 1950-talet. Nu relanserar vi pennan un- der namnet Executive. Ballograf Executive kombinerar tidlös klassisk penndesign, med modern skrivteknik. Det bästa från 50-talet är bevarat i denna penna, med rena linjer och färg- valet som står i smakfull kontrast till överdelen i metall. -

Paperworld 31
paperworld 31. Januar. – 3.Februar 2009 Das offizielle Messemagazin NEWS & SERVICES Hallenplan Hall plan Ausstellerliste List of exhibitors Highlights Product News Trends 2009/2010 TOPICS Lebensstil trifft Schreibstil Lifestyles and writing styles unite Das grüne Büro The green office Halle 3.1 / D61 • Halle 4.2 / E61 topfair LIEBE TOP FAIR LESER! Ob Fachgeschäfte für Papier, Fachbesucher aus aller Welt füllen hier die nach der Advents- und Weihnachtszeit leeren Ladenregale Bürobedarf oder Schreibwaren, wieder auf, entdecken die neuesten Trends und las- Papeterien, Spielwarenhandlungen, sen sich inspirieren, treffen ihre Geschäftspartner, knüpfen neue Kontakte und erfahren, was die Hobby- und Kreativmärkte, Branche aktuell bewegt. Eine in Breite und Tiefe einmalige Produktvielfalt, Aussteller aus aller Welt Computerläden, Papeterien, sowie ein umfangreiches Rahmenprogramm mit Grußkartenshops, Geschenkboutiquen, zahlreichen Sonderschauen, Vortragsarealen und Preisverleihungen machen die Paperworld zum ob Großeinkäufer, Facheinzelhändler, Branchenevent Nummer eins – ich freue mich, dass Sie sich dieses Branchenevent nicht entgehen Ruth Lorenz, Grafiker, Produktdesigner oder lassen. Bereichsleiterin Agenturinhaber – sie alle starten mit Messe Frankfurt Besonders ans Herz legen möchte ich Ihnen einen Exhibition GmbH der Paperworld, der internationalen Besuch der Paperworld-Trendschau in Halle 6.1. Vice President Das bora.herke Stilbüro hat die vier maßgeblichen Leitmesse für Papier, Bürobedarf Messe Frankfurt Trendströmungen der kommenden Saison definiert Exhibition GmbH und Schreibwaren, in das neue und diese in Trendwelten anschaulich umgesetzt – lassen Sie sich überraschen und nehmen Sie wert- Geschäftsjahr. volle Tipps und Anregungen für die eigene Laden- und Schaufenstergestaltung mit. Ebenfalls nicht verpassen sollten Sie das PBS-Forum in Halle 3.0. Branchenexperten geben hier wertvolles Wissen für den Berufsalltag weiter, von dem Sie garantiert profitieren werden. -
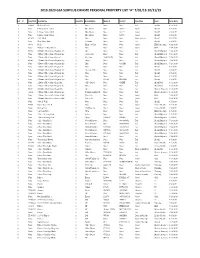
2019-2020 Gsa Surplus Ewaste Personal Property List "A" 7/01/19-10/31/19
2019-2020 GSA SURPLUS EWASTE PERSONAL PROPERTY LIST "A" 7/01/19-10/31/19 Lot # Asset ID # Description Quantity Brand/Make Model # Serial # Condition Dept Form Date 1 #10403 Desktop Hutch 1 None None None Fair Auditor 6/18/2019 1 None L-Shape Corner Desk 1 Blue Shield None 52599 Good Sheriff 8/5/2019 1 None L-Shape Corner Desk 1 Blue Shield None 52579 Good Sheriff 8/5/2019 1 None L-Shape Corner Desk 1 Blue Shield None 52578 Good Sheriff 8/5/2019 1 #11429 Table Desk 1 None None None Broken-Frame Sheriff 8/5/2019 1 None Chair/Floor Mat 1 Velostat None None Fair Sheriff 8/5/2019 1 #8449 Desk 1 Knape & Vogt None None Good IHSS-Pub. Auth. 8/22/2019 1 None 4 Drawer,2 Door Hutch 1 None None None Good GSA 9/24/2019 2 #5036 4 Drawer File Cabinet-Regular Size 1 Hon None None Fair Sheriff-Dispatch 7/24/2019 2 None 4 Drawer File Cabinet-Regular Size 1 1000 Series None None Fair Sheriff-Dispatch 7/24/2019 2 None 3 Drawer File Cabinet-Lateral 1 Meridian 26-4220-3N None Fair Sheriff-Dispatch 7/24/2019 2 #5045 7 Drawer File Cabinet-Regular Size 1 Holga None None Fair Sheriff-Dispatch 7/24/2019 2 None 4 Drawer File Cabinet-Regular Size 1 Hon None C1GYSR Fair Sheriff-Dispatch 7/24/2019 2 None 2 Drawer File Cabinet-Regular Size 1 None None None Fair Sheriff 8/5/2019 2 None 3 Drawer File Cabinet-Regular Size 1 Herman Miller None None Fair Sheriff 8/5/2019 2 None 2 Drawer File Cabinet-Regular Size 1 None None None Fair Sheriff 8/5/2019 2 None 4 Drawer File Cabinet-Regular Size 1 None None None Fair Sheriff 8/5/2019 2 None 2 Drawer File Cabinet-Regular Size 1 Hon PF20FF D9G8DN Fair Sheriff 8/5/2019 2 None 2 Drawer File Cabinet-Legal Size 1 Office Depot None CJ4BXF Fair Sheriff-Jail 8/12/2019 2 None 2 Drawer File Cabinet-Regular Size 1 None None None Fair Sheriff-Jail 8/12/2019 2 #5837 4 Drawer File Cabinet-Legal Size 1 Hon None None Fair District Attorney 8/16/2019 2 #3636 5 Drawer File Cabinet-Regular Size 1 Remington Rand R. -

Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #68 - September 2013
Gary & Myrna Lehrer’s Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #68 - September 2013 See the Catalog in full color on the web site. For about a week you’ll need a password for access (be sure to also see what’s remaining from previous Catalogs). WEBSITE PAS SWORD FOR CATALOG #68: (www.gopens.com): BOOK Catalog #68 Features: Featured are Conklin Crescent-fill pens A group of modern LE’s, Ball Pens, Visconti & Omas Some incredible Italian and German Pens! 20+ Manufacturers Represented Montblanc 149 Demonstrator; Namiki White Tiger! Over 275 Items Contact Information: Tel: (203) 389-5295 email: [email protected] Fax: (859) 909-1882 Call until 9:00 PM Eastern Time; Fax or email anytime We check our email often Subscription Expired: A (3) on your mailing label means your subscription has expired. “Internet Only” renewal is $10. “Hard Copy” Renewal is $25 US and $35 Foreign (see website for details). Received a sample copy? Don’t forget to subscribe ! Please see inside front page for abbreviations and other important information! Gary & Myrna Lehrer 16 Mulberry Road Woodbridge, CT 06525-1717 September 2013 - CATALOG #67 Here’s Some Other Important Information : GIFT CERTIFICATES : Available in any denomination. No extra cost! No expiration! Always fully refundable! CONSIGNMENT - PEN PURCHASES : Ask about consignment rates for the Catalog (we reserve the right to turn down consignments), or see the web site for details. We are also always looking to purchase one pen or entire collections. ABBREVIATIONS : Mint - No sign of use Fine - Used, parts show wear Near Mint - Slightest signs of use Good - Well used, imprints may be almost Excellent - Imprints good, writes well, looks great gone, plating wear Fine+ - One of the following: some brassing, Fair - A parts pen some darkening, or some wear ---------------------------------------------------------------------------------------------------------------------------------------- LF - Lever Filler HR - Hard Rubber PF - Plunger Filler (ie. -

Download Catalog #92
Gary & Myrna Lehrer’s Quarterly Illustrated Vintage Pen Catalog [email protected] Issue #92 - April 2020 See the Catalog in full color on the web site. For about a week you’ll need a password for access (be sure to also see what’s remaining from previous Catalogs). WEBSITE PASSWORD FOR CATALOG #92: (www.gopens.com): TREE Catalog #92 Features: Vintage Montblancs Mint-in-Box vintage & modern LEs, pens & pencils Parker 75 and Parker 61 Over 30 different Manufacturers Over 280 Items Contact Information: Tel: (203) 389-5295 email: [email protected] Fax: (419) 730-1479 Call until 8:30 PM Eastern Time; Fax or email anytime We check our email often Subscription Expired: A (92) on your mailing label means your subscription has expired. “Internet Only” renewal is $10. “Hard Copy” Renewal is $25 US and $35 Foreign (see website for details). Received a sample copy? Don’t forget to subscribe! Please see inside front page for abbreviations and other important information! Gary & Myrna Lehrer 16 Mulberry Road Woodbridge, CT 06525-1717 April 2020 - CATALOG #92 Here’s Some Other Important Information: GIFT CERTIFICATES: Available in any denomination. No extra cost! No expiration! CONSIGNMENT - PEN PURCHASES: We usually accept a small number of consignments. Ask about consignment rates (we reserve the right to turn down consignments), or see the website for details. We are also always looking to purchase one pen or entire collections. ABBREVIATIONS: Mint - No sign of use Fine - Used, parts show wear Near Mint - Slightest signs of use Good - Well used, imprints may be almost Excellent - Imprints good, writes well, looks great gone, plating wear Fine+ - One of the following: some brassing, Fair - A parts pen some darkening, or some wear ---------------------------------------------------------------------------------------------------------------------------------------- LF - Lever Fill HR - Hard Rubber VF - Vacuum-Filler (ie. -

CATALOGUE2019.Pdf
Redan 1945 började Ballograf sin produktion i ett garage i Göteborg och idag kan vi stoltsera med att vara Sveriges enda penntillverkare. Genom åren har vi kontinuerligt utvecklat och förbättrat våra produkter, så det är med gott samvete vi lämnar livstids garanti på varje penna som lämnar vår fabrik. Vi arbetar med noggrant utvalda material för att kunna garantera att våra pen- nor håller vad vi lovar och vi har fått utmärkelser för vår genomarbetade design. Våra pennor säljs idag framgångsrikt i bland annat Japan, Schweiz, Finland, Italien, Tyskland, Österrike och USA. De 35 anställa vid fabriken i Göteborg tillverkar varje år över 4 miljoner kulspet- spennor och stiftpennor. The story of Ballograf began back in 1945 in a garage in Gothenburg, and today we’re proud to say that we’re the only Swedish pen manufacturer. Through the years we’ve continuously developed and improved our products, so it is with a clear conscience we leave a lifetime guarantee on every pen that leaves our factory. We work with carefully selected materials to ensure that our pens live up to our high standars and we’ve received awards for our elaborate design. Our pens are currently sold successfully in Japan, Switzerland, Finland, Italy, Germany, Austria and the USA. The 35 employees at the factory in Gothenburg manufactures over 4 million ballpoint pens and mechanical pencils anually. 1 2 ELOX Kulspetspenna & Stiftpenna Ballpoint Pen & Mechanical Pencil Elox är vår mest exklusiva penna. Varje Elox mont- eras för hand i fabriken i Göteborg och självklart levereras den med livstids garanti på tryckmekanismen.