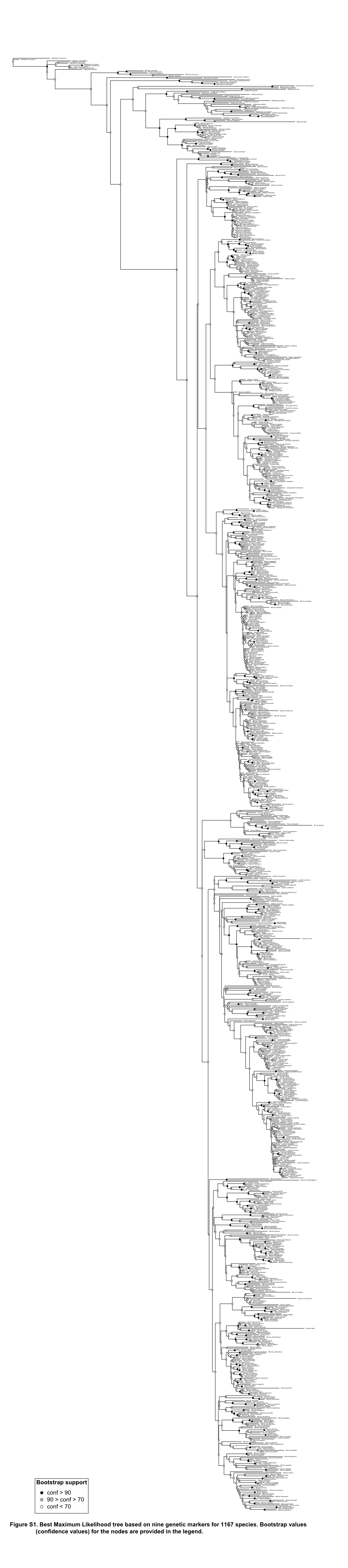
R Graphics Output
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommendation of Native Species for the Reforestation of Degraded Land Using Live Staking in Antioquia and Caldas’ Departments (Colombia)
UNIVERSITÀ DEGLI STUDI DI PADOVA Department of Land, Environment Agriculture and Forestry Second Cycle Degree (MSc) in Forest Science Recommendation of native species for the reforestation of degraded land using live staking in Antioquia and Caldas’ Departments (Colombia) Supervisor Prof. Lorenzo Marini Co-supervisor Prof. Jaime Polanía Vorenberg Submitted by Alicia Pardo Moy Student N. 1218558 2019/2020 Summary Although Colombia is one of the countries with the greatest biodiversity in the world, it has many degraded areas due to agricultural and mining practices that have been carried out in recent decades. The high Andean forests are especially vulnerable to this type of soil erosion. The corporate purpose of ‘Reforestadora El Guásimo S.A.S.’ is to use wood from its plantations, but it also follows the parameters of the Forest Stewardship Council (FSC). For this reason, it carries out reforestation activities and programs and, very particularly, it is interested in carrying out ecological restoration processes in some critical sites. The study area is located between 2000 and 2750 masl and is considered a low Andean humid forest (bmh-MB). The average annual precipitation rate is 2057 mm and the average temperature is around 11 ºC. The soil has a sandy loam texture with low pH, which limits the amount of nutrients it can absorb. FAO (2014) suggests that around 10 genera are enough for a proper restoration. After a bibliographic revision, the genera chosen were Alchornea, Billia, Ficus, Inga, Meriania, Miconia, Ocotea, Protium, Prunus, Psidium, Symplocos, Tibouchina, and Weinmannia. Two inventories from 2013 and 2019, helped to determine different biodiversity indexes to check the survival of different species and to suggest the adequate characteristics of the individuals for a successful vegetative stakes reforestation. -

The Evolution of Bat Pollination: a Phylogenetic Perspective
Annals of Botany 104: 1017–1043, 2009 doi:10.1093/aob/mcp197, available online at www.aob.oxfordjournals.org INVITED REVIEW The evolution of bat pollination: a phylogenetic perspective Theodore H. Fleming1,*, Cullen Geiselman2 and W. John Kress3 1Emeritus, Department of Biology, University of Miami, Coral Gables, FL 33124, USA, 2Institute of Systematic Botany, The New York Botanical Garden, Bronx, NY 10458, USA and 3Department of Botany, MRC-166, National Museum of Natural History, Smithsonian Institution, PO Box 37012, Washington, DC 20013-7012, USA Received: 2 April 2009 Returned for revision: 27 May 2009 Accepted: 13 July 2009 Published electronically: 29 September 2009 † Background Most tropical and subtropical plants are biotically pollinated, and insects are the major pollinators. A small but ecologically and economically important group of plants classified in 28 orders, 67 families and about 528 species of angiosperms are pollinated by nectar-feeding bats. From a phylogenetic perspective this is a derived pollination mode involving a relatively large and energetically expensive pollinator. Here its ecologi- cal and evolutionary consequences are explored. Downloaded from † Scope and Conclusions This review summarizes adaptations in bats and plants that facilitate this interaction and discusses the evolution of bat pollination from a plant phylogenetic perspective. Two families of bats contain specialized flower visitors, one in the Old World and one in the New World. Adaptation to pollination by bats has evolved independently many times from a variety of ancestral conditions, including insect-, bird- and non-volant mammal-pollination. Bat pollination predominates in very few families but is relatively common in certain angiosperm subfamilies and tribes. -

Molecular Phylogenetics of Melastomataceae and Memecylaceae: Implications for Character Evolution1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Access LMU American Journal of Botany 88(3): 486±498. 2001. MOLECULAR PHYLOGENETICS OF MELASTOMATACEAE AND MEMECYLACEAE: IMPLICATIONS FOR CHARACTER EVOLUTION1 G. CLAUSING2,4 AND S. S. RENNER3,4 2Institut fuÈr Spezielle Botanik, UniversitaÈt Mainz, D-55099 Mainz, Germany; 3Department of Biology, University of Missouri-St. Louis, 8001 Natural Bridge Rd., St. Louis, Missouri 63121 USA; and The Missouri Botanical Garden, St. Louis, Missouri 63166 USA Melastomataceae are among the most abundant and diversi®ed groups of plants throughout the tropics, but their intrafamily rela- tionships and morphological evolution are poorly understood. Here we report the results of parsimony and maximum likelihood (ML) analyses of cpDNA sequences from the rbcL and ndhF genes and the rpl16 intron, generated for eight outgroups (Crypteroniaceae, Alzateaceae, Rhynchocalycaceae, Oliniaceae, Penaeaceae, Myrtaceae, and Onagraceae) and 54 species of melastomes. The sample represents 42 of the family's currently recognized ;150 genera, the 13 traditional tribes, and the three subfamilies, Astronioideae, Melastomatoideae, and Memecyloideae (5 Memecylaceae DC.). Parsimony and ML yield congruent topologies that place Memecy- laceae as sister to Melastomataceae. Pternandra, a Southeast Asian genus of 15 species of which ®ve were sampled, is the ®rst- branching Melastomataceae. This placement has low bootstrap support (72%), but agrees with morphological treatments that placed Pternandra in Melastomatacaeae because of its acrodromal leaf venation, usually ranked as a tribe or subfamily. The interxylary phloem islands found in Memecylaceae and Pternandra, but not most other Melastomataceae, likely evolved in parallel because Pternandra resembles Melastomataceae in its other wood characters. -

Biota Colombiana ISSN: 0124-5376 [email protected] Instituto De Investigación De Recursos Biológicos "Alexander Von Humboldt" Colombia
Biota Colombiana ISSN: 0124-5376 [email protected] Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Colombia Calderón Sáenz, Eduardo; Mendoza Cifuentes, Humberto Melastomatáceas de los Géneros Axinaea, Blakea, Castratella, Centronia, Killipia, Meriania, Monochaetum, Ossaea y Tibouchina en Colombia Biota Colombiana, vol. 1, núm. 3, diciembre, 2000, pp. 336-357 Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Bogotá, Colombia Disponible en: http://www.redalyc.org/articulo.oa?id=49110301 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Biota336- ColombianaMelastomataceae 1 (3) 336 - de357, Colombia 2000 Calderón-Sáenz & Mendoza-Cifuentes Melastomatáceas de los Géneros Axinaea, Blakea, Castratella, Centronia, Killipia, Meriania, Monochaetum, Ossaea y Tibouchina en Colombia Eduardo Calderón-Sáenz1 y Humberto Mendoza-Cifuentes2 1 Avenida 3CN # 62N-77, Villas de San Martín, Casa 43, Cali - Colombia. [email protected] 2 Instituto Alexander von Humboldt, Calle 37 # 8-40, Mezzanine, Bogotá D.C. - Colombia. [email protected] Palabras Clave: Melastomataceae, Lista de Especies, Colombia Las melastomatáceas no han recibido ningún trata- destacado ocupa la obra del botánico colombiano José Je- miento que incluya las especies conocidas para Colombia o rónimo Triana (1873), publicada en Inglaterra, y quien de al menos para una parte de sus géneros. Los únicos com- todas formas tuvo como referencia los trabajos de Karsten pendios específicos son los de Uribe (1972, 1976 y 1983). -

1 Updates Required to Plant Systematics: A
Updates Required to Plant Systematics: A Phylogenetic Approach, Third Edition, as a Result of Recent Publications (Updated June 13, 2014) As necessitated by recent publications, updates to the Third Edition of our textbook will be provided in this document. It is hoped that this list will facilitate the efficient incorporation new systematic information into systematic courses in which our textbook is used. Plant systematics is a dynamic field, and new information on phylogenetic relationships is constantly being published. Thus, it is not surprising that even introductory texts require constant modification in order to stay current. The updates are organized by chapter and page number. Some require only minor changes, as indicated below, while others will require more extensive modifications of the wording in the text or figures, and in such cases we have presented here only a summary of the major points. The eventual fourth edition will, of course, contain many organizational changes not treated below. Page iv: Meriania hernandii Meriania hernandoi Chapter 1. Page 12, in Literature Cited, replace “Stuessy, T. F. 1990” with “Stuessy, T. F. 2009,” which is the second edition of this book. Stuessy, T. F. 2009. Plant taxonomy: The systematic evaluation of comparative data. 2nd ed. Columbia University Press, New York. Chapter 2. Page 37, column 1, line 5: Stuessy 1983, 1990;… Stuessy 1983, 2009; … And in Literature Cited, replace “Stuessy 1990” with: Stuessy, T. F. 2009. Plant taxonomy: The systematic evaluation of comparative data. 2nd ed. Columbia University Press, New York. Chapter 4. Page 58, column 1, line 5: and Dilcher 1974). …, Dilcher 1974, and Ellis et al. -

Presentación De Powerpoint
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/336231014 DIVERSIDAD DE LA FAMILIA MELASTOMATACEAE EN ECUADOR Presentation · October 2018 DOI: 10.13140/RG.2.2.36690.09922 CITATIONS READS 0 980 2 authors, including: Diana Fernández Fernández Instituto Nacional de Biodiversidad (INABIO), Ecuador 45 PUBLICATIONS 122 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Plantas de los Páramos del Distrito Metropolitano de Quito, Ecuador View project A new species of Sloanea (Elaeocarpaceae) subgenus Quadrisepala from Ecuadorian Amazonia View project All content following this page was uploaded by Diana Fernández Fernández on 03 October 2019. The user has requested enhancement of the downloaded file. DIVERSIDAD DE LA FAMILIA MELASTOMATACEAE EN ECUADOR Diana Fernández Fernández1* Carmen Ulloa Ulloa2 1Instituto Nacional de Biodiversidad, Herbario QCNE, *[email protected] 2Missouri Botanical Garden Quito, 25 de octubre del 2018 Foto: W. Palacios Miconia yeseniae W. Palacios, D. Fernández & Michelang., sp. nov. Melastomataceae Distribución: Zonas húmedas tropicales y subtropicales de América, África y Asia. Subfamilias: • Olisbeoideae • Melastomatoideae (Neotrópico) Neotrópico : • 3700 especies • 180 géneros • 7 tribus www.tropicos.org Melastomataceae en Ecuador Nᵒ Tribus Nᵒ Géneros Nᵒ Especies Nᵒ Endémicas Autores Jorgensen & León Yánez, 6 43 553 199 1999 Ulloa & Neill, 2011; 6 43 588 183 Penneys & Cotton, 2011 Fernández & Ulloa, 2017 6 41 610 200 (inédito) Tibouchina oroensis Gleason Importancia del estudio de la familia Melastomataceae en Ecuador • Tercera familia más diversa (3%) • Se encuentra desde los bosques de tierras bajas hasta los páramos, excepto es los matorrales y bosques secos. -

Meriania Mendoza.Af Anales 69(2).Qxd 18/12/2012 17:24 Página 259
2317_Meriania_Mendoza.af_Anales 69(2).qxd 18/12/2012 17:24 Página 259 Anales del Jardín Botánico de Madrid 69(2): 259-294, julio-diciembre 2012. ISSN: 0211-1322. doi: 10.3989/ajbm. 2317 Novedades en Centronia y Meriania (Merianieae, Melastomataceae) y revisión taxonómica de Meriania grupo brachycera Humberto Mendoza-Cifuentes1* & José Luis Fernández-Alonso2 1Programa de Postgrado en Sistemática, Instituto de Ciencias Naturales, A. A. 7495, Universidad Nacional de Colombia, Bogotá D.C., Colombia; [email protected] 2Real Jardín Botánico RJB-CSIC, Plaza de Murillo 2, E-28014 Madrid, España; [email protected] Resumen Abstract Mendoza-Cifuentes, H. & Fernández-Alonso, J.L. 2012. Novedades en Mendoza-Cifuentes, H. & Fernández-Alonso, J.L. 2012. Novelties in Cen- Centronia y Meriania (Merianieae, Melastomataceae) y revisión taxonómi- tronia and Meriania (Merianieae, Melastomataceae) and a taxonomic revi- ca de Meriania grupo brachycera. Anales Jard. Bot. Madrid 69(2): 259-294. sion of Meriania brachycera group. Anales Jard. Bot. Madrid 69(2): 259- 294 (In Spanish). Tomando como base un análisis filogenético del género Centronia D. Don A historical review, the circumscription problems and nomenclatural fundamentado en caracteres morfológicos, se presenta una síntesis histó- changes based upon a phylogenetic morphological analysis of the genus rica del género, se documentan sus problemas de circunscripción con Centronia are presented. In this framework, four Andean species of Cen- otros géneros de Merianieae y se proponen los cambios nomenclaturales tronia are transfered to the genus Meriania (M. brachycera, M. haeman- que es necesario realizar. Se transfieren cuatro especies andinas del géne- tha, M. mutabilis and M. mutisii) and five new species related to this group ro Centronia a Meriania Sw. -

Novedades En Centronia Y Meriania (Merianieae, Melastomataceae) Y Revisión Taxonómica De Meriania Grupo Brachycera
2317_Meriania_Mendoza.af_Anales 69(2).qxd 18/12/2012 17:24 Página 259 Anales del Jardín Botánico de Madrid 69(2): 259-294, julio-diciembre 2012. ISSN: 0211-1322. doi: 10.3989/ajbm. 2317 Novedades en Centronia y Meriania (Merianieae, Melastomataceae) y revisión taxonómica de Meriania grupo brachycera Humberto Mendoza-Cifuentes1* & José Luis Fernández-Alonso2 1Programa de Postgrado en Sistemática, Instituto de Ciencias Naturales, A. A. 7495, Universidad Nacional de Colombia, Bogotá D.C., Colombia; [email protected] 2Real Jardín Botánico RJB-CSIC, Plaza de Murillo 2, E-28014 Madrid, España; [email protected] Resumen Abstract Mendoza-Cifuentes, H. & Fernández-Alonso, J.L. 2012. Novedades en Mendoza-Cifuentes, H. & Fernández-Alonso, J.L. 2012. Novelties in Cen- Centronia y Meriania (Merianieae, Melastomataceae) y revisión taxonómi- tronia and Meriania (Merianieae, Melastomataceae) and a taxonomic revi- ca de Meriania grupo brachycera. Anales Jard. Bot. Madrid 69(2): 259-294. sion of Meriania brachycera group. Anales Jard. Bot. Madrid 69(2): 259- 294 (In Spanish). Tomando como base un análisis filogenético del género Centronia D. Don A historical review, the circumscription problems and nomenclatural fundamentado en caracteres morfológicos, se presenta una síntesis histó- changes based upon a phylogenetic morphological analysis of the genus rica del género, se documentan sus problemas de circunscripción con Centronia are presented. In this framework, four Andean species of Cen- otros géneros de Merianieae y se proponen los cambios nomenclaturales tronia are transfered to the genus Meriania (M. brachycera, M. haeman- que es necesario realizar. Se transfieren cuatro especies andinas del géne- tha, M. mutabilis and M. mutisii) and five new species related to this group ro Centronia a Meriania Sw. -

The Evolution of Bat Pollination: a Phylogenetic Perspective
Annals of Botany Page 1 of 27 doi:10.1093/aob/mcp197, available online at www.aob.oxfordjournals.org INVITED REVIEW The evolution of bat pollination: a phylogenetic perspective Theodore H. Fleming1,*, Cullen Geiselman2 and W. John Kress3 1Emeritus, Department of Biology, University of Miami, Coral Gables, FL 33124, USA, 2Institute of Systematic Botany, The New York Botanical Garden, Bronx, NY 10458, USA and 3Department of Botany, MRC-166, National Museum of Natural History, Smithsonian Institution, PO Box 37012, Washington, DC 20013-7012, USA Received: 2 April 2009 Returned for revision: 27 May 2009 Accepted: 13 July 2009 † Background Most tropical and subtropical plants are biotically pollinated, and insects are the major pollinators. A small but ecologically and economically important group of plants classified in 28 orders, 67 families and about 528 species of angiosperms are pollinated by nectar-feeding bats. From a phylogenetic perspective this is a derived pollination mode involving a relatively large and energetically expensive pollinator. Here its ecologi- cal and evolutionary consequences are explored. † Scope and Conclusions This review summarizes adaptations in bats and plants that facilitate this interaction and discusses the evolution of bat pollination from a plant phylogenetic perspective. Two families of bats contain specialized flower visitors, one in the Old World and one in the New World. Adaptation to pollination by bats has evolved independently many times from a variety of ancestral conditions, including insect-, bird- and non-volant mammal-pollination. Bat pollination predominates in very few families but is relatively common in certain angiosperm subfamilies and tribes. We propose that flower-visiting bats provide two important benefits to plants: they deposit large amounts of pollen and a variety of pollen genotypes on plant stigmas and, compared with many other pollinators, they are long-distance pollen dispersers. -
2-EVALUACIÓN DE CARACTERES.P65
BOTÁNICA EVALUACIÓN DE CARACTERES DEL CÁLIZ Y DE LOS ESTAMBRES EN LA TRIBU MERIANIEAE (MELASTOMATACEAE) Y DEFINICIÓN DE HOMOLOGÍAS Por Humberto Mendoza-Cifuentes1 & José Luis Fernández-Alonso1,2 Resumen H. Mendoza-Cifuentes & J. L. Fernández-Alonso: Evaluación de caracteres del cáliz y de los estambres en la tribu Merianieae (Melastomataceae) y definición de homologías. Rev. Acad. Colomb. Cienc. 34 (131): 143-172, 2010. ISSN 0370-3908. Los caracteres del cáliz y de los estambres se han utilizado clásicamente para diferenciar géneros y establecer secciones dentro de géneros en la tribu Merianieae (Melastomataceae), pero no se han analizado integralmente desde el punto de vista de homologías. Se estableció una lista completa de especies para la tribu y se evaluó la variación de los caracteres del cáliz y los estam- bres. En total se registran 15 géneros y 277 especies en Merianieae, el 78% de las cuales fueron evaluadas completamente y el resto de ellas parcialmente en lo referente a los caracteres estudia- dos. Se establecieron 23 caracteres, cinco asociados al cáliz y 18 a los estambres. En Merianieae se presenta gran variación en el desarrollo de los lóbulos y del diente dorsal del cáliz y en la apertura de la caliptra. Las flores son diplostémonas en todos los géneros. Se encontró que en cinco de los géneros (el 80% de las especies de la tribu) los estambres son no geniculados. Se plantea que la ausencia de geniculación en los estambres se originó por fusión de tejidos del conectivo y del filamento, que impide que los estambres se descontorsionen en la antesis. -

Divergence Times, Historical Biogeography, and Shifts in Speciation Rates of Myrtales Q ⇑ Brent A
Molecular Phylogenetics and Evolution 95 (2016) 116–136 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Divergence times, historical biogeography, and shifts in speciation rates of Myrtales q ⇑ Brent A. Berger a,b, , Ricardo Kriebel b, Daniel Spalink b, Kenneth J. Sytsma b a Department of Biological Sciences, St. John’s University, 8000 Utopia Parkway, Queens, NY 11432, USA b Department of Botany, University of Wisconsin-Madison, 430 Lincoln Dr., Madison, WI 53706, USA article info abstract Article history: We examine the eudicot order Myrtales, a clade with strong Gondwanan representation for most of its Received 9 June 2015 families. Although previous phylogenetic studies greatly improved our understanding of intergeneric Revised 3 September 2015 and interspecific relationships within the order, our understanding of inter-familial relationships still Accepted 4 October 2015 remains unresolved; hence, we also lack a robust time-calibrated chronogram to address hypotheses Available online 14 November 2015 (e.g., biogeography and diversification rates) that have implicit time assumptions. Six loci (rbcL, ndhF, matK, matR, 18S, and 26S) were amplified and sequenced for 102 taxa across Myrtales for phylogenetic Keywords: reconstruction and ten fossil priors were utilized to produce a chronogram in BEAST. Combretaceae is BEAST identified as the sister clade to all remaining families with moderate support, and within the latter clade, BioGeoBEARS BAMM two strongly supported groups are seen: (1) Onagraceae + Lythraceae, and (2) Melastomataceae + the Diversification Crypteroniaceae, Alzateaceae, Penaeaceae clade along with Myrtaceae + Vochysiaceae. Divergence time Gondwana estimates suggest Myrtales diverged from Geraniales 124 Mya during the Aptian of the Early Phylogenetics Cretaceous. -

A Guide to Curating New World Melastomataceae Collections with a Linear Generic Sequence to World-Wide Melastomataceae
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 9 October 2020 doi:10.20944/preprints202010.0203.v1 A guide to curating New World Melastomataceae collections with a linear generic sequence to world-wide Melastomataceae Fabián A. Michelangeli1, Frank Almeda2, Renato Goldenberg3, Darin S. Penneys4 1Institute of Systematic Botany, New York Botanical Garden, 2900 Southern Blvd, Bronx, NY 10458-5126 USA; https://orcid.org/0000-0001-7348-143X; [email protected] 2California Academy of Sciences, Institute for Biodiversity Science and Sustainability, Department of Botany, 55 Music Concourse Drive, Golden Gate Park, San Francisco, CA 94118-4599, USA; https://orcid.org/0000-0001-5091-6875; [email protected] 3Departamento de Botânica, Universidade Federal do Paraná, R. Francisco H. dos Santos, s.n., Campus do C. Politécnico, Curitiba 81531-980 BRAZIL; https://orcid.org/0000-0002-7047- 6720; [email protected] 4University of North Carolina Wilmington, Department of Biology and Marine Biology, 601 S. College Rd., Wilmington, NC, 28403, USA; https://orcid.org/0000-0003-0727-2829; [email protected] Abstract: The following guide is aimed at aiding in the curation of herbarium collections in Melastomataceae, with an emphasis on the New World species. It contains a summary of the taxonomic realignments at the tribal and generic level within Neotropical taxa of Melastomataceae, as well as some general comments for other groups. A table with a generic linear sequence is also provided, as well as tables with new and synonymized New World genera since 2005 and all currently accepted species. Lastly, a table with the synonyms of over 1000 accepted Neotropical species that have been impacted by these generic realignments is also provided.