Bioassessment of Freshwater Lentic Wetlands Using the Reference Condition Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Using Specimens from the Past to Understand the Living World Through Digitization
Using specimens from the past to understand the living world through digitization Drs. Jessica L. Ware, William Kuhn, Dirk Gassmann and John Abbott Rutgers University, Newark Dragonflies: Order Odonata Suborders Anisoptera (unequal wings): Dragonflies ~3000 species Anisozygoptera ~3 species Zygoptera: Damselflies ~3000 species Perchers, Fliers, Migrators, & Homebodies Dragonfly flight Wing venation affects wing camber, lift, and ultimately flight patterns Dragonfly flight Stiffness varies along length of the wing with vein density and thickness Dragonfly flight Certain wing traits are correlated with specific flight styles Dragonfly collections: invaluable treasures Collection name # spp. #specimens Florida State Collection 2728 150K Ware Lab Collection 373 4K Smithsonian Collection 253 200K M.L. May Collection 300 10K Dragonfly collections: invaluable treasures Harness information in collections Targeted Odonata Wing Digitization (TOWD) project TOWD project scanning protocol TOWD project scanning protocol TOWD project TOWD project TOWD project 10 10 TOWD project More information at: https://willkuhn.github.io/towd/ Aspect ratios: How elongate is the wing compared to its overall area? • High Aspect ratio Low Aspect ratio Long narrow wings, Wing Short broad wings, optimized for: Wing optimized long-distance flight for: Maneuverability, turning High Aspect Low Aspect Ratio Ratio More data on aspect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months work More data on apsect ratios, better interpretations? 2007: Hand measured forewings of 85 specimens, 7 months 2019: Odomatic measurements for 206 work specimens, 2-3 minutes of work! Are there differences in aspect ratios? Perchers have significantly lower aspect ratios than fliers. The p-value is .001819. The result is significant at p < .05. -
![[The Pond\. Odonatoptera (Odonata)]](https://docslib.b-cdn.net/cover/4965/the-pond-odonatoptera-odonata-114965.webp)
[The Pond\. Odonatoptera (Odonata)]
Odonatological Abstracts 1987 1993 (15761) SAIKI, M.K. &T.P. LOWE, 1987. Selenium (15763) ARNOLD, A., 1993. Die Libellen (Odonata) in aquatic organisms from subsurface agricultur- der “Papitzer Lehmlachen” im NSG Luppeaue bei al drainagewater, San JoaquinValley, California. Leipzig. Verbff. NaturkMus. Leipzig 11; 27-34. - Archs emir. Contam. Toxicol. 16: 657-670. — (US (Zur schonen Aussicht 25, D-04435 Schkeuditz). Fish & Wildl. Serv., Natn. Fisheries Contaminant The locality is situated 10km NW of the city centre Res. Cent., Field Res, Stn, 6924 Tremont Rd, Dixon, of Leipzig, E Germany (alt, 97 m). An annotated CA 95620, USA). list is presented of 30 spp., evidenced during 1985- Concentrations of total selenium were investigated -1993. in plant and animal samplesfrom Kesterson Reser- voir, receiving agricultural drainage water (Merced (15764) BEKUZIN, A.A., 1993. Otryad Strekozy - — Co.) and, as a reference, from the Volta Wildlife Odonatoptera(Odonata). [OrderDragonflies — km of which Area, ca 10 S Kesterson, has high qual- Odonatoptera(Odonata)].Insectsof Uzbekistan , pp. ity irrigationwater. Overall,selenium concentrations 19-22,Fan, Tashkent, (Russ.). - (Author’s address in samples from Kesterson averaged about 100-fold unknown). than those from Volta. in and A rather 20 of higher Thus, May general text, mentioning (out 76) spp. Aug. 1983, the concentrations (pg/g dry weight) at No locality data, but some notes on their habitats Kesterson in larval had of 160- and vertical in Central Asia. Zygoptera a range occurrence 220 and in Anisoptera 50-160. In Volta,these values were 1.2-2.I and 1.1-2.5, respectively. In compari- (15765) GAO, Zhaoning, 1993. -
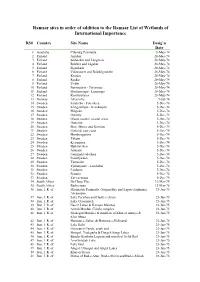
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Conospermum Hookeri Hookeri (Tasmanian Smokebush)
Listing Statement for Conospermum hookeri (tasmanian smokebush) Conospermum hookeri tasmanian smokebush FAMILY: Proteaceae T A S M A N I A N T H R E A T E N E D S P E C I E S L I S T I N G S T A T E M E N T GROUP: Dicotyledon Photos: Naomi Lawrence Scientific name: Conospermum hookeri (Meisn.) E.M.Benn., Fl. Australia 16: 485 (1995) (Meisn.) Common name: tasmanian smokebush Name history: previously known in Tasmania as Conospermum taxifolium. Group: vascular plant, dicotyledon, family Proteaceae Status: Threatened Species Protection Act 1995: vulnerable Environment Protection and Biodiversity Conservation Act 1999: Vulnerable Distribution: Biogeographic origin: endemic to Tasmania Tasmanian NRM regions: North, South Tasmanian IBRA Bioregions (V6): South East, Northern Midlands, Ben Lomond, Flinders Figure 1. Distribution of Conospermum hookeri Plate 1. Conospermum hookeri in flower. showing IBRA (V6) bioregions 1 Threatened Species Section – Department of Primary Industries, Parks, Water and Environment Listing Statement for Conospermum hookeri (tasmanian smokebush) Conospermum hookeri may be limited by low seed SUMMARY: Conospermum hookeri (tasmanian production rates. Other species of Conospermum smokebush) is a small shrub in the Proteaceae are known to have low reproductive outputs. family. It is endemic to Tasmania, occurring Approximately 50% of flowers of Conospermum along the East Coast from Bruny Island to species form fruit though only a small Cape Barren Island in 10 locations, two proportion of these produce viable seed presumed locally extinct and another of (Morrison et al. 1994). uncertain status. The number of subpopulations is estimated to be 40, with five Conospermum hookeri makes a highly significant presumed locally extinct or of uncertain status. -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM Y Y Department of Primary Industries, Parks, Water and Environment (DPIPWE) GPO Box 44 HOBART Tasmania 7001 Designation date Site Reference Number Australia Ph: +61 3 6233 8011 2. Date this sheet was completed/updated: July 2012 3. Country: Australia 4. Name of the Ramsar site: The precise name of the designated site in one of the three official languages (English, French or Spanish) of the Convention. -

Draft Index of Keys
Draft Index of Keys This document will be an update of the taxonomic references contained within Hawking 20001 which can still be purchased from MDFRC on (02) 6024 9650 or [email protected]. We have made the descision to make this draft version publicly available so that other taxonomy end-users may have access to the information during the refining process and also to encourage comment on the usability of the keys referred to or provide information on other keys that have not been reffered to. Please email all comments to [email protected]. 1Hawking, J.H. (2000) A preliminary guide to keys and zoological information to identify invertebrates form Australian freshwaters. Identification Guide No. 2 (2nd Edition), Cooperative Research Centre for Freshwater Ecology: Albury Index of Keys Contents Contents ................................................................................................................................................. 2 Introduction ............................................................................................................................................. 8 Major Group ............................................................................................................................................ 8 Minor Group ................................................................................................................................................... 8 Order ............................................................................................................................................................. -

3966 Tour Op 4Col
The Tasmanian Advantage natural and cultural features of Tasmania a resource manual aimed at developing knowledge and interpretive skills specific to Tasmania Contents 1 INTRODUCTION The aim of the manual Notesheets & how to use them Interpretation tips & useful references Minimal impact tourism 2 TASMANIA IN BRIEF Location Size Climate Population National parks Tasmania’s Wilderness World Heritage Area (WHA) Marine reserves Regional Forest Agreement (RFA) 4 INTERPRETATION AND TIPS Background What is interpretation? What is the aim of your operation? Principles of interpretation Planning to interpret Conducting your tour Research your content Manage the potential risks Evaluate your tour Commercial operators information 5 NATURAL ADVANTAGE Antarctic connection Geodiversity Marine environment Plant communities Threatened fauna species Mammals Birds Reptiles Freshwater fishes Invertebrates Fire Threats 6 HERITAGE Tasmanian Aboriginal heritage European history Convicts Whaling Pining Mining Coastal fishing Inland fishing History of the parks service History of forestry History of hydro electric power Gordon below Franklin dam controversy 6 WHAT AND WHERE: EAST & NORTHEAST National parks Reserved areas Great short walks Tasmanian trail Snippets of history What’s in a name? 7 WHAT AND WHERE: SOUTH & CENTRAL PLATEAU 8 WHAT AND WHERE: WEST & NORTHWEST 9 REFERENCES Useful references List of notesheets 10 NOTESHEETS: FAUNA Wildlife, Living with wildlife, Caring for nature, Threatened species, Threats 11 NOTESHEETS: PARKS & PLACES Parks & places, -

Caring for Our Country Achievements
caring for our country Achievements Report COASTAL ENVIRONMENTS AND CRITICAL AQUATIC HABITATS 2008 –2013 Coastwest, community seagrass monitoring project, Roebuck Bay, Broome, Western Australia. Source: Environs Kimberley Coastal Environments and Critical Aquatic Habitats Coastal Environments and Critical Aquatic Habitats Fragile ecosystems are being protected and rehabilitated by improving water quality, protecting Ramsar wetlands and delivering the Great Barrier Reef Rescue package. Coastwest, community seagrass monitoring project, Roebuck Bay, Broome, Western Australia. Source: Environs Kimberley 3 Table of contents Introduction 6 Reef Rescue outcomes 9 Outcome 1 Reduce the discharge of dissolved nutrients and chemicals from agricultural lands to the Great Barrier Reef lagoon by 25 per cent. 9 Outcome 2 Reduce the discharge of sediments and nutrients from agricultural lands to the Great Barrier Reef lagoon by 10 per cent 9 Case study: Minimal soil disturbance in cane farming—Tully/Murray catchment, Queensland 10 Case study: Repairing bank erosion in the Upper Johnstone catchment, Queensland 12 Case study: Sugar cane partnerships, Mackay Whitsunday region, Queensland 13 Case study: Horticulturalists nurturing the reef, Mackay Whitsunday region, Queensland 14 Case study: Land and Sea Country Indigenous Partnerships Program, Queensland 15 Outcome 3.1 Deliver actions that sustain the environmental values of priority sites in the Ramsar estate, particularly sites in northern and remote Australia. 17 Case study: Currawinya Lakes Ramsar wetland, Queensland 18 Case study: Macquarie Marshes Ramsar wetland, New South Wales 22 Case study: Interlaken Ramsar wetland, Tasmania 23 Case study: Peel–Yalgorup System Ramsar wetland, Western Australia 25 Outcome 3.2 Deliver actions that sustain the environmental values of an additional 25 per cent of (non-Ramsar) priority coastal and inland high conservation value aquatic ecosystems [now known as high ecological value aquatic ecosystems] including, as a priority, sites in the Murray–Darling Basin. -

Extent and Impacts of Dryland Salinity in Tasmania
National Land and Water Resources Audit Extent and impacts of Dryland Salinity in Tasmania Project 1A VOLUME 2 - APPENDICES C.H. Bastick and M.G. Walker Department of Primary Industries, Water and Environment August 2000 DEPARTMENT of PRIMARY INDUSTRIES, WATER and ENVIRONMENT LIST OF APPENDICES Appendix 1 LAND SYSTEMS IN TASMANIA CONTAINING AREAS OF SALINITY................................................................1 Appendix 2 EXTENT, TRENDS AND ECONOMIC IMPACT OF DRYLAND SALINITY IN TASMANIA...................................16 Appendix 3 GROUND WATER ....................................................................28 Appendix 4(a) SURFACE WATER MONITORING .......................................32 Appendix 4(b) SURFACE WATER IN TASMANIA - An Overview ..............36 Appendix 5 POTENTIAL IMPACTS OF SALINITY ON BIODIVERSITY VALUES IN TASMANIA.............................46 Appendix 1 LAND SYSTEMS IN TASMANIA CONTAINING AREAS OF SALINITY 1 LAND SYSTEMS IN TASMANIA CONTAINING AREAS OF SALINITY · The Soil Conservation section of the Tasmanian Department of Agriculture between 1980 and 1989 carried out a series of reconnaissance surveys of the State's land resources. · It was intended that the data collected would allow a more rational approach to Tasmania's soil conservation problems and serve as a base for further studies, such as the detailed mapping of land resources in selected areas and the sampling of soils for physical and chemical analyses. It was therefore decided that this would be a logical framework on which to develop the Dryland Salinity Audit process. · Land resources result from the interaction of geology, climate, topography, soils and vegetation · Areas where these are considered to be relatively uniform for broad scale uses are classified as land components. Land components are grouped into larger entities called land systems, which are the mapping units used for this Audit. -

Identification Guide to the Australian Odonata Australian the to Guide Identification
Identification Guide to theAustralian Odonata www.environment.nsw.gov.au Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW National Library of Australia Cataloguing-in-Publication data Theischinger, G. (Gunther), 1940– Identification Guide to the Australian Odonata 1. Odonata – Australia. 2. Odonata – Australia – Identification. I. Endersby I. (Ian), 1941- . II. Department of Environment and Climate Change NSW © 2009 Department of Environment, Climate Change and Water NSW Front cover: Petalura gigantea, male (photo R. Tuft) Prepared by: Gunther Theischinger, Waters and Catchments Science, Department of Environment, Climate Change and Water NSW and Ian Endersby, 56 Looker Road, Montmorency, Victoria 3094 Published by: Department of Environment, Climate Change and Water NSW 59–61 Goulburn Street Sydney PO Box A290 Sydney South 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131555 (information & publication requests) Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. ISBN 978 1 74232 475 3 DECCW 2009/730 December 2009 Printed using environmentally sustainable paper. Contents About this guide iv 1 Introduction 1 2 Systematics -

Ramsar COP8 DOC. 6 Report of the Secretary General Pursuant To
"Wetlands: water, life, and culture" 8th Meeting of the Conference of the Contracting Parties to the Convention on Wetlands (Ramsar, Iran, 1971) Valencia, Spain, 18-26 November 2002 Ramsar COP8 DOC. 6 Report of the Secretary General pursuant to Article 8.2 (b), (c), and (d) concerning the List of Wetlands of International Importance 1. Article 8.2 of the Convention states that: “The continuing bureau duties [the Ramsar Bureau, or convention secretariat] shall be, inter alia : … b) to maintain the List of Wetlands of International Importance and to be informed by the Contracting Parties of any additions, extensions, deletions or restrictions concerning wetlands included in the List provided in accordance with paragraph 5 of Article 21; c) to be informed by the Contracting Parties of any changes in the ecological character of wetlands included in the List provided in accordance with paragraph 2 of Article 32; d) to forward notification of any alterations to the List, or changes in character of wetlands included therein, to all Contracting Parties and to arrange for these matters to be discussed at the next Conference; e) to make known to the Contracting Party concerned, the recommendations of the Conferences in respect of such alterations to the List or of changes in the character of wetlands included therein.” 2. The present report of the Secretary General conveys to the 8th Meeting of the Conference of the Parties the information requested under Article 8 concerning the List of Wetlands of International Importance since the closure of -

Critical Species of Odonata in Australia
---Guardians of the watershed. Global status of Odonata: critical species, threat and conservation --- Critical species of Odonata in Australia John H. Hawking 1 & Gunther Theischinger 2 1 Cooperative Research Centre for Freshwater Ecology, Murray-Darling Freshwater Research Centre, PO Box 921, Albury NSW, Australia 2640. <[email protected]> 2 Environment Protection Authority, New South Wales, 480 Weeroona Rd, Lidcombe NSW, Australia 2141. <[email protected]> Key words: Odonata, dragonfly, IUCN, critical species, conservation, Australia. ABSTRACT The Australian Odonata fauna is reviewed. The state of the current taxonomy and ecology, studies on biodiversity, studies on larvae and the all identification keys are reported. The conservation status of the Australian odonates is evaluated and the endangered species identified. In addition the endemic species, species with unusual biology and species, not threatened yet, but maybe becoming critical in the future are discussed and listed. INTRODUCTION Australia has a diverse odonate fauna with many relict (most endemic) and most of the modern families (Watson et al. 1991). The Australian fauna is now largely described, but the lack of organised surveys resulted in limited distributional and ecological information. The conservation of Australian Odonata also received scant attention, except for Watson et al. (1991) promoting the awareness of Australia's large endemic fauna, the listing of four species as endangered (Moore 1997; IUCN 2003) and the suggesting of categories for all Australian species (Hawking 1999). This conservation report summarizes the odonate studies/ literature for species found in Continental Australia (including nearby smaller and larger islands) plus Lord Howe Island and Norfolk Island. Australia encompasses tropical, temperate, arid, alpine and off shore island climatic regions, with the land mass situated between latitudes 11-44 os and 113-154 °E, and flanked on the west by the Indian Ocean and on the east by the Pacific Ocean.