The State of Hamilton County Lakes: a 25-Year Perspective 1993 - 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Piseco Lake's Least Wanted
Spiny Water Flea Invasive zooplankton found in Piseco Lake, Lake Pleasant, and Sacandaga Lake. Does not harm humans or pets. Gobbles up native zooplankton. Competes with native fish for food. Clumps on fishing line, prevents fish from being landed. No control methods exist. EWM Eurasian Watermilfoil Variable Leaf Milfoil Aquatic invasive plants. EWM is found in Lake Algonquin, Fifth, Sixth and Seventh Lakes. VLM is found in Eldon Lake, Forked Lake, Fourth, Fifth, Sixth and Seventh Lakes, Lake Durant, Long Lake, Raquette Lake, and Rock Pond. Inhibit boating and fishing, and devalue shoreline property. Expensive to control. VLM Check and remove visible plants, animals, and mud from your boat, trailer, and equipment. Dispose of in the trash or on dry land. Drain water from bilge, motor, livewells, bait buckets, ballast tanks, kayaks, and canoes. Clean your watercraft and gear with hot (greater than 140oF) water or spray with high pressure water. Dry at least 5 days. 518-548-3991 · www.hcswcd.com Facebook · YouTube Map of Adirondack Lakes with aquatic invaders. From the Adirondack Park Invasive Plant Program P.O. Box 65 Keene Valley, New York 12943 (518) 576-2082 http://adkinvasives.com/ List of Adirondack Waters with Aquatic Invaders. From the Adirondack Park Invasive Plant Program P.O. Box 65 Keene Valley, New York 12943 (518) 576-2082 http://adkinvasives.com/ Over. List of Adirondack Waters with Aquatic Invaders. From the Adirondack Park Invasive Plant Program P.O. Box 65 Keene Valley, New York 12943 (518) 576-2082 http://adkinvasives.com/ . -

Freshwater Fishing: a Driver for Ecotourism
New York FRESHWATER April 2019 FISHINGDigest Fishing: A Sport For Everyone NY Fishing 101 page 10 A Female's Guide to Fishing page 30 A summary of 2019–2020 regulations and useful information for New York anglers www.dec.ny.gov Message from the Governor Freshwater Fishing: A Driver for Ecotourism New York State is committed to increasing and supporting a wide array of ecotourism initiatives, including freshwater fishing. Our approach is simple—we are strengthening our commitment to protect New York State’s vast natural resources while seeking compelling ways for people to enjoy the great outdoors in a socially and environmentally responsible manner. The result is sustainable economic activity based on a sincere appreciation of our state’s natural resources and the values they provide. We invite New Yorkers and visitors alike to enjoy our high-quality water resources. New York is blessed with fisheries resources across the state. Every day, we manage and protect these fisheries with an eye to the future. To date, New York has made substantial investments in our fishing access sites to ensure that boaters and anglers have safe and well-maintained parking areas, access points, and boat launch sites. In addition, we are currently investing an additional $3.2 million in waterway access in 2019, including: • New or renovated boat launch sites on Cayuga, Oneida, and Otisco lakes • Upgrades to existing launch sites on Cranberry Lake, Delaware River, Lake Placid, Lake Champlain, Lake Ontario, Chautauqua Lake and Fourth Lake. New York continues to improve and modernize our fish hatcheries. As Governor, I have committed $17 million to hatchery improvements. -
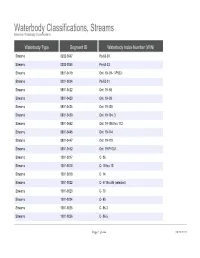
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

Fulton Chain Wild Forest Unit Management Plan
DEC Publication FULTON CHAIN WILD FOREST UNIT MANAGEMENT PLAN · .. JANUARY ·1990 NEW YORK STATE DEPARTMENT. OF ENVIRONMENTAL. Co·NSERVATION FULTON CHAIN WILD FOREST UNIT MANAGEMENT PLAN New York State Department of Environmental conservation Mario Cuomo Thomas Jorling Governor Commissioner TO: The Record /, J FROM: Thomas C. Jorl~V RE: Unit Management Plan Fulton Chain Wild Forest The Unit Management Plan for the Fulton Chain Wild Forest has been completed. It is consistent with the guidelines and criteria of the Adirondack Park State Land Master Plan involved citizen participation, is consistent with the State Constitution, the Environmental Conservation Law, rules, regulations and policy. The Plan includes management objectives for a five-year period and is hereby approved and adopted. cc: L. Marsh Task Force Leader: D. V. Gray, Herkimer Region 6 staff Contributors: E. Smith - Wildlife L. Blake, s. Gray III, R. McKinley T. Voss - Wildlife s. Coutant, J. Manion, J. Dexter, M. Ayers - Wildlife P. Hartmann, D. Riedman, T. Perkins, w. Gordon - Fisheries M. Gleason, c. Bunn, J. Kramer - Lands J. Hasse - Fisheries and Forests R. Van Wie - Operations c. Munger - Administration R. Dawson - Operations c. Slater - Operations L. Maley - Law Enforcement Central Office Contributors: M. Baldwin, D. Perham, L. Sweet, P. Bach, B. Rihm - Lands and Forests Region 5 staff Contributors: J. English, B. Finlayson - Lands and Forests. i FULTON CHAIN WILD FOREST Unit Management Plan The Fulton Chain Wild Forest is a picturesque land of rolling woodlands, rocky hills, wetlands, lakes, ponds, and beaver meadows nestled within a mix of State and private owner- ships. It includes part of the beautiful Fulton Chain of Lakes and is rich in local Adirondack history. -

Synopsis of the Parasites of Fishes of Canada
1 ci Bulletin of the Fisheries Research Board of Canada DFO - Library / MPO - Bibliothèque 12039476 Synopsis of the Parasites of Fishes of Canada BULLETIN 199 Ottawa 1979 '.^Y. Government of Canada Gouvernement du Canada * F sher es and Oceans Pëches et Océans Synopsis of thc Parasites orr Fishes of Canade Bulletins are designed to interpret current knowledge in scientific fields per- tinent to Canadian fisheries and aquatic environments. Recent numbers in this series are listed at the back of this Bulletin. The Journal of the Fisheries Research Board of Canada is published in annual volumes of monthly issues and Miscellaneous Special Publications are issued periodically. These series are available from authorized bookstore agents, other bookstores, or you may send your prepaid order to the Canadian Government Publishing Centre, Supply and Services Canada, Hull, Que. K I A 0S9. Make cheques or money orders payable in Canadian funds to the Receiver General for Canada. Editor and Director J. C. STEVENSON, PH.D. of Scientific Information Deputy Editor J. WATSON, PH.D. D. G. Co«, PH.D. Assistant Editors LORRAINE C. SMITH, PH.D. J. CAMP G. J. NEVILLE Production-Documentation MONA SMITH MICKEY LEWIS Department of Fisheries and Oceans Scientific Information and Publications Branch Ottawa, Canada K1A 0E6 BULLETIN 199 Synopsis of the Parasites of Fishes of Canada L. Margolis • J. R. Arthur Department of Fisheries and Oceans Resource Services Branch Pacific Biological Station Nanaimo, B.C. V9R 5K6 DEPARTMENT OF FISHERIES AND OCEANS Ottawa 1979 0Minister of Supply and Services Canada 1979 Available from authorized bookstore agents, other bookstores, or you may send your prepaid order to the Canadian Government Publishing Centre, Supply and Services Canada, Hull, Que. -

Piseco Lake Public Campgrounds Unit Management Plan
Division of Operations Bureau of Recreation __________________________________________ Piseco Lake Public Campgrounds Unit Management Plan Final Plan Town of Arietta, Hamilton County, New York _____________________________________________ October 2017 New York State Department of Environmental Conservation Division of Operations, 3rd Floor 625 Broadway, Albany, NY12233 Governor ANDREW M. CUOMO Commissioner BASIL SEGGOS OFFICE OF THE COMMISSIONER New York State Department of Environmental Conserviltion 625 Broadway, 14th Floor, Albany, New York 12233-1010 P: (518) 402-8545 I F: (518) 402-8541 MEMORANDUM TO: The Record FROM: Basil Seggos SUBJECT: Piseco Lake Campground The Piseco Lake Campground Unit Management Plan has been completed. The Adirondack Park Agency has found the UMP to be in conformance with the Adirondack Park State Land Master Plan. The UMP is consistent with Environmental Conservation Law, and Department Rules, Regulations and Policies and is hereby approved and adopted. Basil Seggos Commissioner New York State Department of Environmental Conservation 11)/,, I ' 1--- Dcpartmentof r--"~ St-AEWYORKHl)f I c._, ':"'•o•••Nrn- Environmental ~ Conservation PISECO LAKE PUBLIC CAMPGROUNDS SITE SPECIFIC - VOLUME II UNIT MANAGEMENT PLAN NOTE: Volume I is a generic plan and contains overview, environmental setting, goals, policy, management, and impact assessment criteria which pertain universally and in common to all Adirondack and Catskill public campgrounds and special day-use classified intensive-use areas. Volume II is a site- specific document containing inventories of physical, biological, and human-made features, together with specific management actions for the individual site. Volume III contains support data in the form of an appendix to Volumes I and II. The New York State Department of Environmental Conservation (DEC) prepares unit management plans (UMP) to cover the next five-year management period. -

Warmwater Fishing New York's Adirondack Lakes
Warmwater Fishing New York’s Adirondack Lakes New York State is full of good waters to catch a variety of warmwater fish species. Here is just a sampling of some of the lakes located in New York’s Adirondack Park. Size rentals boat boat cabin rentalscabin Waterbody (acres) County bass largemouth smallmouth bass pike northern musky musky/tiger pickerel walleye yellow perch bullhead crappie sunfish bass rock white perch trout/salmon ice fishing bait/tackle nearby boat rampspublic boat ramps private boatcartop access marinas campsites Blake Reservoir 710 St. Lawrence * * * * * * * * * Blue Mountain Lake 1,260 Hamilton * * * * * * * * * * * Brant Lake 1,300 Warren * * * * * * * * * * * * * Carry Falls Reservoir 6,458 St. Lawrence * * * * * * * * * * Chazy Lake 1,606 Clinton * * * * * * * * * Cranberry Lake 6,987 St. Lawrence * * * * * * * * * * * Eagle Lake 422 Essex * * * * * * * * * * * * * Eighth Lake 314 Hamilton * * * * * * * * * * Forked Lake 1,250 Hamilton * * * * * * * * * * Fourth Lake 2,137 Herkimer * * * * * * * * * * * * * * Great Sacandaga Lake 22,500 Fulton/ * * * * * * * * * * * * * * * * * * * * Saratoga Higley Flow 698 St. Lawrence * * * * * * * * * * * * Indian Lake 4,364 Hamilton * * * * * * * * * * * * * * * * Lake George 28,000 multiple * * * * * * * * * * * * * * * * * * * Lake Placid 2,803 Essex * * * * * * * * * * * * * Lake Pleasant 1,400 Hamilton * * * * * * * * * * * * * * * * * * Lincoln Pond 500 Essex * * * * * * * * * * Long Lake 4,090 Hamilton * * * * * * * * * * * * * * * * Loon Lake 600 Warren * * * * * * * -

NBC Transmitter.
m NATIONAL EfiOADCASTINQ COMPANY, general library 30 ROCKEFELLER PLAZA, NEW YORK, N. 1 >:.-s Vr-. iS- ’ NBC VOL. 6 JANUARY, 1940 No. 1 LATEST PROGRESS IN TELEVISION NEW YEAR SftS MANY TRAINING FCC VIEWS NEW PORTABLE UNIT GROUPS HELD FOR YOUNGER MEN elevision de- S the New Year ajrproaches and gets underway, it finds T velops so rapid- AI the largest number yet of employe training courses ly that it is always in action. This is a result of the Company’s policy of filling outmoding its own vacancies from its own ranks. It has been said more and news. This month more often in the past few years that the Company is old there are several enough to prepare its personnel to fill the responsible posi- items for the record. tions created or opened as time goes on, and this year a We are all familiar more comprehensve effort than ever is being made in that with the ten-ton, direction. two-truck mobile Ashton Dunn of Personnel has already organized a group unit which has so for the purpose of learning the structure and activities of successfully picked various departments. It is similar to last year’s group which up such nemos as was developed to satisfy the expressed interest of the younger Evolution of an Idea. boxing and tennis employes. Some of the more specialized courses recently matches, and base- planned or begun are working in connection with the larger ball and football games. This sleek monster is the incredi- group to fill out the general training program. -

Adirondack Watershed Institute Stewardship Program Summary of Programs and Research 2014
n1 fAdirondack Watershed Institute Stewardship AdirondackProgram Summary Watershed of Programs Institute Stewardshipand Research Program 2014 Summary of Programs and Research 2014 Adirondack Watershed Institute Report # PSCAWI 2015-02 ADIRONDACK WATERSHED INSTITUTE THE YEAR IN REVIEW 2 STEWARDSHIP PROGRAM The Year in Review Aquatic invasive species (AIS) continue to be a great concern all across the Adirondack region, demanding increasing attention and resources from communities and agencies far and wide. The Adirondack Watershed Institute Stewardship Program (AWISP) is part of coordinated efforts at the local, regional and statewide levels to detect and arrest the spread of AIS. The Adirondack Watershed Institute (AWI) works year-round with partner organizations, communities and government agencies to understand and manage a range of environmental quality issues through research and education. 2014 marked the fifteenth field season for the AWISP. 2014 highlights: Clean, Drain, Dry! AWISP stewards provided coverage at 31 launches on 26 lakes and ponds this season sharing the message of “Clean, Drain, and Dry!” New Education Program! The AWISP launched an off-site environmental education program called the Water Shield Workshop. The program integrates land-based exercises with on-water activities for participants of all ages. Water Shield Workshops were held at Lake Pleasant, Schroon Lake, and Lower Saranac Lake in 2014. Finding Bythotrephes! Survey efforts by AWISP staff confirmed the presence of spiny waterflea (Bythotrephes longimanus) in Lake Pleasant and Piseco Lake for the first time. See it! Touch it! Learn it! The AWISP purchased an Enviroscape watershed model for education and outreach activities. Round-up of accomplishments, by the numbers: AWISP stewards confirmed and removed 834 AIS from inspected watercraft across the Adirondack region. -

Silver Lake Wilderness Unit Management Plan Resolution March 9, 2006 Page 2
New York State Department of Environmental Conservation Division of Lands and Forests SILVER LAKE WILDERNESS AREA Silver Lake Wilderness Sacandaga Primitive Area Cathead Mountain Primitive Area UNIT MANAGEMENT PLAN April 2006 GEORGE E. PATAKI, Governor DENISE M. SHEEHAN, Commissioner THIS PAGE INTENTIONALLY LEFT BLANK Silver Lake Wilderness Area Silver Lake Wilderness Sacandaga Primitive Area Cathead Mountain Primitive Area Unit Management Plan April 2006 New York State Department of Environmental Conservation Division of Lands & Forests 625 Broadway Albany, NY 12233-4254 (518) 473-9518 www.dec.state.ny.us THIS PAGE INTENTIONALLY LEFT BLANK MEMORANDUM TO: The Record FROM: Denise M. Sheehan SUBJECT: Silver Lake Wilderness Area Final Unit Management Plan (Final UMP) The Final UMP for the Silver Lake Wilderness Area Final Unit Management Plan (Final UMP) has been completed. The Final UMP is consistent with the guidelines and criteria of the Adirondack Park State Land Master Plan, the State Constitution, Environmental Conservation Law, and Department rules, regulations and policies. The Final UMP/FEIS includes management objectives and a five year budget and is hereby approved and adopted. RESOLUTION ADOPTED BY THE ADIRONDACK PARK AGENCY WITH RESPECT TO SILVER LAKE WILDERNESS, SACANDAGA PRIMITIVE AND CATHEAD MOUNTAIN PRIMITIVE AREAS UNIT MANAGEMENT PLAN March 9, 2006 WHEREAS, Section 816 of the Adirondack Park Agency Act directs the Department of Environmental Conservation to develop, in consultation with the Adirondack Park Agency, individual -

Watershed Stewardship Program Summary of Programs and Research 2011
Watershed Stewardship Program Summary of Programs and Research 2011 Adirondack Watershed Institute Watershed Stewardship Program Report # AWI 2012-01 2 Watershed Stewardship Program Summary of Programs and Research 2011 Table of Contents Dedication ............................................................................................................................................... 4 Executive Summary and Introduction ...................................................................................................... 5 West Central Adirondack Region Summary ............................................................................................ 17 Watershed Stewardship Program- Staff Profiles .................................................................................... 24 Recreation Use Study: Cranberry Lake State Boat Launch ...................................................................... 30 Recreation Use Study: Fourth Lake State Boat Launch ........................................................................... 38 Recreation Use Study: Lake Flower State Boat Launch ........................................................................... 48 Recreation Use Study: Lake Placid State Boat Launch ............................................................................ 60 Recreation Use Study: Lake Placid Village Launch .................................................................................. 70 Recreation Use Study: Long Lake State Boat Launch ............................................................................. -

Freshwater Fishing Regulation Guide
NEW YORK STATE FRESHWATER FISHING REGULATIONS GUIDE Regulations in efect April 1, 2021 Department of Environmental Conservation New York State has about 300 Complaints are forwarded to an ECO Environmental Conservation Ofcers for investigation. The more detailed (ECOs) and Investigators (ECIs) who work information you provide, the more likely throughout the state, including New the violator will be apprehended. Try to York City. ECOs spend most of their time remember the “who, what, where, when, patrolling within their assigned county. and how" of the event. The assistance of the public is essential • Keep a distance from the violator. Do to the efective enforcement of state not approach or attempt to confront environmental laws and regulations. If you suspects. They may be dangerous, observe someone violating Environmental destroy evidence, or simply evade Conservation Law or see the results of ofcers if forewarned. a violation, REPORT IT! Poachers and • Who did it? Provide names, ages, sex, polluters are thieves, stealing from you, height, weight, clothing or vehicle our fellow anglers, and future generations. descriptions, and other details. Those who pollute our air or water, destroy • What occurred? What exactly do you our environment, or ignore fsh and wildlife think is the nature of the violation? laws are criminals. Examples — taking over limit of fsh, Contact an Environmental snagging, illegal netting, fshing out of season, trespassing. Conservation Police Ofcer • When did it occur? Provide dates and (ECO) times. Is it still in progress, ongoing, or For general questions, call 1-877-457-5680. something yet to happen? Examples— You will speak with a dispatcher who will happening right now, happens every Fri assist you or connect you to an ECO.