Title DRL AR2017 Kala.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skillv/Smanagement
Vol:1 | Issue 3 | October 2014 For Private Circulation Only PAGES 60 ‘Andhra Pradesh gives importance to PPP model for tourism development’ Chandana Khan IAS Special Chief Secretary, Tourism & Culture, Archaeology & Museums, Archives & Youth Services & Sports, NCC, Govt. of AP Page no 45 Page no 24 HYDERABAD Trying to Regain the Lost Ground The Land of Royals and Heritage Page no 20 ‘We are trying to create a global tourism destination through the Spice Route’ P I Sheik Pareeth (IAS) Director, Kerala Tourism Page no 50 Owner-Manager relationship: Page no 10 Are Indian owners becoming assertive? New Executive Committee Members (2014-15) of SIHRA elected during the 63rd AGM, held in Chennai When students decide to step into the hospitality industry, the first thing they do is enroll into an IHM and attain the hospitality management Page no 30 degree. Each year, hundreds of students graduate out of IHMs where students are equipped to be Kerala Tourism: Unveiling managers. Does the hospitality industry really need so many managers or do they need skill- Hidden Treasures based professionals? Page no 38 Skill v/sManagement Need to revisit our priorities Page no 48 SIHRA NEWS | OCTOBER 2014 3 EDITOR & COO* Sheldon Santwan Head-Editorial Operations & Special Projects Content Sumit Jha Assistant Editor P Krishna Kumar 07 | Editor's Desk EDITORIAL BOARD - SIHRA Honorary Secretary 08 | News Round-up T Nataraajan Advisor 16 | FH RAI Annual Convention 2014: R Rangachari Secretary General An effort to make the Industry Voice Elina Maller Heard EDITORIAL -

Activity Report 2009 – 2010
Activity Report 2009 – 2010 L V Prasad Eye Institute Kallam Anji Reddy Campus L V Prasad Marg, Banjara Hills Hyderabad 500 034, India Tel: 91 40 3061 2345 Fax: 91 40 2354 8271 e-mail: [email protected] L V Prasad Eye Institute Patia, Bhubaneswar 751 024 Orissa, India Tel: 91 0674 3989 2020 Fax: 91 0674 3987 130 e-mail: [email protected] L V Prasad Eye Institute G M R Varalakshmi Campus Door No: 11-113/1 Hanumanthawaka Junction Visakhapatnam 530 040 Andhra Pradesh, India Tel: 91 0891 3989 2020 Fax: 91 0891 398 4444 L V Prasad Eye Institute e-mail: [email protected] Excellence • Equity • Effi ciency Art with vision, for vision Artist-in-residence Sisir Sahana in his workshop on A view of the Art Gallery on Level 6 at Hyderabad LVPEI’s Kallam Anji Reddy campus, Hyderabad creating campus, where several works by Mr Surya Prakash, one of his signature glass sculptures. Inset: A piece from our senior artist-in-residence are on display. his latest collection, entitled “The long climb”. Inset: The hand that wields the paintbrush! L V Prasad Eye Institute Committed to excellence and equity in eye care Activity Report April 2009 – March 2010 Collaborating Centre for Prevention of Blindness L V Prasad Eye Institute, a not-for-profi t charitable organization, is governed by two trusts: Hyderabad Eye Institute and Hyderabad Eye Research Foundation. Donations to Hyderabad Eye Research Foundation are 175% exempt under section 35 (i) (ii) and donations made to Hyderabad Eye Institute are 50% exempt under section 80G of the Income Tax Act. -
View Annual Report
CONTENTS CHAIRMAN’S LETTER DEAR SHAREHOLDERS FY2012 has been a good year for your Company. The key financial results were: ¥ Consolidated revenues increased by 30% to Rs. 96.7 billion in FY2012. ¥ Earnings before interest, taxes, depreciation and amortization (EBITDA)1 rose by 55% to Rs. 25.4 billion. ¥ Profit after Tax (PAT)2 grew by 45% to Rs. 15.3 billion. ¥ Diluted Earnings per Share (EPS) increased from Rs. 64.9 in FY2011 to Rs. 83.8 in FY2012. I am particularly delighted by four developments. First, your Company succeeded in yet another blockbuster generic launch in the USA under 180- days marketing exclusivity. Dr. Reddy’s launched olanzapine 20 mg tablets, the generic version of the brand Zyprexa®. Olanzapine is used to treat schizophrenia and bipolar disorder. This product has added around USD 100 million to your Company’s revenues for FY2012. Second, the biosimilars business continues along its impressive growth path. In my letter to you last year, I had discussed the critical importance of developing biosimilars in the years to come. I am happy to note that your Company’s global biosimilars business grew by 45% over last year and recorded sales of USD 26 million. Today, the biosimilars portfolio of Dr. Reddy’s constitutes (i) filgrastim, (ii) peg-filgrastim, (iii) rituximab and (iv) darbepoetin alfa, which have commercial presence in 13 countries among emerging markets. These are helping to treat patients suffering from cancer — and at prices that are significantly more affordable than the corresponding innovator drugs. Soon, I expect to see Dr. Reddy’s biosimilars entering developed markets. -

An Exploration of the Institutions, Characteristics and Drivers of Elite Philanthropy in India
Swinburne University of Technology Faculty of Business & Law DRAFT An exploration of the institutions, characteristics and drivers of elite philanthropy in India John Godfrey BA, MSc, Grad. Dip Arts Admin Student ID 1700367 Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy. Swinburne University of Technology, Faculty of Business & Law. Year of approval for award 2020. Abstract This thesis fills a gap in the empirical study of elite philanthropy which to date has been confined to mostly Western philanthropy, informed by Christian and Jewish norms and traditions. This research explores a tradition and practice of philanthropy which has its early roots in dharmic and Islamic tradition yet, as is shown, has been influenced by Western rules and norms. Twenty-eight philanthropists were interviewed. The two religions these respondents most identified with were Hindu or Parsi, though some identified as Jain, Muslim, Christian or Buddhist. The research applies a theoretical framework described as a moral citizenship. This framework brings together two theoretical models developed by Schervish - identification theory and moral biography (Schervish et al. 1998; Schervish and Havens 1997; Schervish and Havens 2001b, 2002; Schervish and Herman 1988; Schervish et al. 2001). These models, they argue, provide the most fruitful way to identify the social and psychological frameworks that mobilise the agency of philanthropists. The methodology used to apply this theoretical framework used long interviews in which respondents were given free rein to talk about their philanthropic activity in their own terms. This methodology follows in the steps of others such as Breeze and Lloyd (2013), Lloyd (2004), Odendahl (1990), Ostrower (1995), Panas (1984, 2019 [1984]), Scaife et al. -

Cancer Kills Indian Drug Pioneer, Anji Reddy, at 72
Cancer kills Indian drug pioneer, Anji Reddy, at 72 18 March 2013 | News | By BioSpectrum Bureau Bangalore: Dr Kallam Anji Reddy, the founder and chairman of the $2 billion Indian pharma giant, Dr Reddy's Laboratories (DRL), died of cancer in Hyderabad at the age of 72 on March 15, 2013. Dr Reddy's mission in life was to provide innovative new medicines at a price that the common man could afford. His passion for research is evident from the fact that Dr Reddy's Laboratories became the first pharmaceutical company of India to initiate basic drug discovery research in 1993. He obtained his BSc in pharmaceutical science and fine chemicals from Bombay University and his PhD in chemical engineering from National Chemical Laboratory, Pune. He served in the state-owned IDPL before he founded DRL in 1984. Dr Reddy also set-up the Institute of Life Sciences and in 1998, set up the Naandi Foundation as a charitable trust. Naandi is probably India's largest rural safe drinking water provider, and gives midday meals to 1.3 million government school-going children and farmers. He also spearheaded and founded the Neo Natal Intensive Care and Emergencies called NICE Foundation, the only institute for newborns in Asia. Dr Reddy won several eminent accolades throughout his career, the most prominent being the Padma Bhushan (one of the highest civilian awards in India), which was awarded to him by the Government of India in April 2011 in recognition of his distinguished service of high order in the field of trade and industry. -

Iam Delighted to Present the Annual Report Of
From the Director’s Desk am delighted to present the Annual Report of the &Communications were planned. One may recall that Indian Statistical Institute for the year 2018-19. This on June 29, 2017, the then Hon’ble President of India, I Institute that started its journey in December 1931 in Shri Pranab Mukherjee, had inaugurated the 125th Birth Kolkata has today grown into a unique institution of higher Anniversary Celebrations of Mahalanobis. learning spread over several cities of India. The Institute, founded by the visionary PC Mahalanobis, continues It is always a delight to inform that once again the its glorious tradition of disseminating knowledge in Institute faculty members have been recognized both Statistics, Mathematics, Computer Science, Quantitative nationally and internationally with a large number of Economics and related subjects. The year 2018-19 saw honors and awards. I mention some of these here. In the formation of the new Council of the Institute. I am 2018, Arunava Sen was conferred the TWAS-Siwei Cheng delighted to welcome Shri Bibek Debroy as the President Prize in Economics and Sanghamitra Bandyopadhyay of the Institute. It is also a privilege that Professor was conferred the TWAS Prize Engineering Sciences in Goverdhan Mehta continues to guide the Institute as the Trieste, Italy. Arup Bose was selected as J.C. Bose Fellow Chairman of the Council. for 2019-2023 after having completed one term of this fellowship from 2014 to 2018. Nikhil Ranjan Pal was The Institute conducted its 53rd Convocation in January appointed President, IEEE Computational Intelligence 2019. The Institute was happy to have Lord Meghnad Society. -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Sun, 26 Sep 2021 17:25:46 GMT) CTRI Number CTRI/2019/05/019097 [Registered on: 13/05/2019] - Trial Registered Prospectively Last Modified On 25/02/2020 Post Graduate Thesis No Type of Trial Interventional Type of Study Biological Study Design Randomized, Parallel Group, Multiple Arm Trial Public Title of Study Comparing the Efficacy and Safety of Xlucane Versus Lucentis® in Patients requiring treatment of wet AMD Scientific Title of Xplore: A Phase III Double-Blind, Parallel Group, Multicenter Study to Compare the Efficacy and Study Safety of Xlucane versus Lucentis® in Patients with Neovascular Age-Related Macular Degeneration Secondary IDs if Any Secondary ID Identifier 2018-002930-19 EudraCT XBR1001 Version 2.0 dated 27 Nov 2018 Protocol Number Details of Principal Details of Principal Investigator Investigator or overall Name Dr Prabhati Mukherji Trial Coordinator (multi-center study) Designation Associate Medical Director Affiliation Kendle India Pvt. Ltd. Address Building No 14, Tower B, 14th Floor, DLF Cyber City, Phase III, Gurgaon HARYANA 122001 India Phone Fax Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr Prabhati Mukherji Query) Designation Associate Medical Director Affiliation Kendle India Pvt. Ltd. Address Building No 14, Tower B, 14th Floor, DLF Cyber City, Phase III, HARYANA 122001 India Phone Fax Email [email protected] Details -

Dr Kallam Anji Reddy: the Man Who Introduced India to the Drug Called Innovation
Dr Kallam Anji Reddy: The man who introduced India to the drug called innovation By Gauri Kamath Dr Kallam Anji Reddy, founder of Hyderabad-based drugmaker Dr Reddy's Laboratories (DRL) and pioneering pharma entrepreneur who passed away on March 15, loved a challenge. This son of a turmeric farmer from Andhra Pradesh was in his element when faced with a seemingly intractable problem or hopeless cause. Whether it was discovering a breakthrough new drug for diabetes in his company's labs, or supplying safe drinking water to rural India through Naandi, the philanthropic organisation that he helped found, Reddy was always ready to pick up the gauntlet. Life did full justice to his enterprising spirit — it threw several challenges his way all of which he gamely tackled. What stood Reddy in good stead were his incurable optimism, his passion, his trust-and-delegate management style, and his ability to continuously seek inspiration. Imitation to Innovation Of the first two there is ample evidence through his career. In the 1980s, when the homegrown private sector was not the dominant force in pharmaceuticals that it is today, his fledgling drug company embarked on the road to reverse-engineer the best-known drugs of western multinationals (MNCs) at a fraction of their prices. He went after the most tough-to-produce ones; his first choice was blood pressure drug methyldopa. The innovator, US-based Merck, whom Reddy hoped to supply to, believed no Indian company could pull it off. Reddy was alien to such pessimism. His team hunkered down to the task and DRL eventually became the MNC's biggest supplier of the drug. -

Zone Wise List of NASI Fellows
The National Academy of Sciences, India (The Oldest Science Academy of India) Zone wise list of Fellows & Honorary Fellows (2021) 5, Lajpatrai Road, Prayagraj – 211002, UP, India 1 The list has been divided into six zones; and each zone is further having the list of scientists of Physical Sciences and Biological Sciences, separately. 2 The National Academy of Sciences, India 5, Lajpatrai Road, Prayagraj – 211002, UP, India Zone wise list of Fellows Zone 1 (Bihar, Jharkhand, Odisha, West Bengal, Meghalaya, Assam, Mizoram, Nagaland, Arunachal Pradesh, Tripura, Manipur and Sikkim) (Section A – Physical Sciences) ACHARYA, Damodar, Chairman, Advisory Board, SOA Deemed to be University, Khandagiri Squre, Bhubanesware - 751030; ACHARYYA, Subhrangsu Kanta, Emeritus Scientist (CSIR), 15, Dr. Sarat Banerjee Road, Kolkata - 700029; ADHIKARI, Satrajit, Sr. Professor of Theoretical Chemistry, School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja SC Mullick Road, Jadavpur, Kolkata - 700032; ADHIKARI, Sukumar Das, Formerly Professor I, HRI,Ald; Professor & Head, Department of Mathematics, Ramakrishna Mission Vivekananda University, Belur Math, Dist Howrah - 711202; BAISNAB, Abhoy Pada, Formerly Professor of Mathematics, Burdwan Univ.; K-3/6, Karunamayee Estate, Salt Lake, Sector II, Kolkata - 700091; BANDYOPADHYAY, Sanghamitra, Professor & Director, Indian Statistical Institute, 203, BT Road, Kolkata - 700108; BANERJEA, Debabrata, Formerly Sir Rashbehary Ghose Professor of Chemistry,CU; Flat A-4/6,Iswar Chandra Nibas 68/1, Bagmari Road, Kolkata - 700054; BANERJEE, Rabin, Emeritus Professor, SN Bose National Centre for Basic Sciences, Block - JD, Sector - III, Salt Lake, Kolkata - 700098; BANERJEE, Soumitro, Professor, Department of Physical Sciences, Indian Institute of Science Education & Research, Mohanpur Campus, WB 741246; BANERJI, Krishna Dulal, Formerly Professor & Head, Chemistry Department, Flat No.C-2,Ramoni Apartments, A/6, P.G. -

Activity Report 2013–2014
ACTIVITY REPORT 2013–2014 L V Prasad Eye Institute Copyright © 2014 All rights reserved EDITORS: Dr Sreedevi Yadavalli, Aravind Chandarlapati DESIGN: Kartheek Koyinni, Y Yedukondalu ASSISTANCE: V Srinivasa Raju, G Rekha Sruthi, N Jayalaxmi and Lakshmi Sakuntala PHOTOGRAPHY: SBN Chary and Sandeep Roy; LVPEI Archives; Grateful thanks to Vicky Roy DONOR RELATIONS: Sam J Balasundaram PRINTERS: TOTEM Advertising & PR Pvt. Ltd. Department of Communications Level 4 L V Prasad Eye Institute Kallam Anji Reddy Campus L V Prasad Marg, Banjara Hills Hyderabad - 500034, India Ph: +91 40 30612445, +91 40 30612446 Email: [email protected], [email protected] Contents The LVPEI Network 02 The Year at a Glance 04 Our Team 06 Boards of Management 08 Foreword 09 Awards and Honours 10 Breaking New Ground 13 Network News 16 Campus News 22 Kallam Anji Reddy Campus, Hyderabad Bhubaneswar Campus GMR Varalakshmi Campus, Visakhapatnam Kode Venkatadri Chowdary Campus, Vijayawada Patient Care Services 44 Vision Rehabilitation 50 Eye Banking 58 Product Development 62 Gullapalli Pratibha Rao International Centre for Advancement of Rural Eye care 64 Academy of Eye Care Education 78 Prof Brien Holden Eye Research Centre 93 Alumni News 121 Our Support 122 Abbreviations Used KAR - Kallam Anji Reddy Campus RIEB - Ramayamma International Eye Bank GMRV - GMR Varalakshmi Campus GPR ICARE - Gullapalli Pratibha Rao International KVC - Kode Venkatadri Chowdary Campus Centre for Advancement of Rural Eye care THE LVPEI NETWORK Centre of Excellence Centre of Excellence -
Seat Matrix for Web Udation
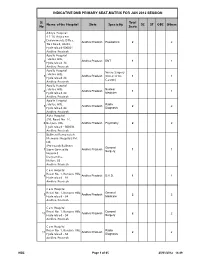
INDICATIVE DNB PRIMARY SEAT-MATRIX FOR JAN 2012 SESSION Sl. Total Name of the Hospital State Speciality SC ST OBC Others No Seats Aditya Hospital 4-1-16, Adjacent Endowments Office, 1 Andhra Pradesh Paediatrics 2 2 Tilak Road, Abids, Hyderabad-500001 Andhra Pradesh Apollo Hospital Jubilee Hills, 2 Andhra Pradesh ENT 1 1 Hyderabad-34 Andhra Pradesh Apollo Hospital Neuro Surgery Jubilee Hills, 3 Andhra Pradesh (Direct 6 Yrs 1 1 Hyderabad-34 Course) Andhra Pradesh Apollo Hospital Jubilee Hills, Nuclear 4 Andhra Pradesh 1 1 Hyderabad-34 Medicine Andhra Pradesh Apollo Hospital Jubilee Hills, Radio 5 Andhra Pradesh 2 2 Hyderabad-34 Diagnosis Andhra Pradesh Asha Hospital 298, Road No. 14, 6 Banjara Hills, Andhra Pradesh Psychiatry 2 2 Hyderabad - 500034 Andhra Pradesh Bollineni Ramanaiah Memorial Hospitals Pvt Ltd, (Previously Bollineni General 7 Super Speciality Andhra Pradesh 1 1 Surgery Hospital) Dargamitta, Nellore-03 Andhra Pradesh Care Hospital Road No. 1, Banjara Hills, 8 Andhra Pradesh D.V.D. 1 1 Hyderabad - 34 Andhra Pradesh Care Hospital Road No. 1, Banjara Hills, General 9 Andhra Pradesh 2 2 Hyderabad - 34 Medicine Andhra Pradesh Care Hospital Road No. 1, Banjara Hills, General 10 Andhra Pradesh 2 2 Hyderabad - 34 Surgery Andhra Pradesh Care Hospital Road No. 1, Banjara Hills, Radio 11 Andhra Pradesh 2 2 Hyderabad - 34 Diagnosis Andhra Pradesh NBE Page 1 of 65 20/01/2012 14:49 INDICATIVE DNB PRIMARY SEAT-MATRIX FOR JAN 2012 SESSION Sl. Total Name of the Hospital State Speciality SC ST OBC Others No Seats Care Hospital The Instt. of Medical Sciences, A.S. -

List of Empanelled Hospital As Per City 28 May 15 0
LIST OF EMPANELLED HOSPITALS Ser No Name of Hospital/Diag Address Phone/Mob/Email Regional Centre City Approved Date of MOA Vaild Recognized for Status of hospital Status of hospital nostic/Dental Centre by MoD Signing up to as per MoA as per Govt letter MOA 1 Agartala Hospital & Research 9A, Mantribari Road, Agartala, GUWAHATI Agartala 27-May-05 27-Nov-05 26-Nov-07 General Medicine, ENT, Dental, General Surgery, Opthalmology, -- NON NABH NON NABH Centre Pvt Ltd, Tripura Obstetrics & Gynaecology, Paediatrics, Pathology and Radio West-799001 Diagnosis. 2 Senapati Dental Lab SDS, Dr BB Senapati C/O Mr Bivek Bojoy, Proprietor, 0381-2221621, GUWAHATI Agartala 27-May-05 1-Apr-10 31-Mar-11 Dental. -- NON NABH NON NABH BB Senapati Retd Law 9436124448, Emaol: Secretary , Master Para [email protected] 3 Teresa Diagnostic Centre, Achintya Bhattacharya TDC, Achintya Bhattacharya, Proprietor, GUWAHATI Agartala 27-May-05 1-Jun-11 31-May-12 Pathology and Radio diagnosis. Radio Diagnosis & Imaging - CT Scan. NON NABH NON NABH 07 Hosp Road, Agartala, 0381-2302717, 9436120567, Tripura, West, Email: [email protected] Pin : 799001 4 Dr Lal Path labs, PB Das Memorial Diagnostic Ph-0381-2319781, 2319782, GUWAHATI Agartala 22-Dec-08 Microbiology, Pathology. -- NON NABH NON NABH Centre, Bidurkarta Chowmani, Mob-9862219897 Agartala-799001 5 Scientific Pathology, Durga Complex Hriparvat AgraTele – 05622151111 LUCKNOW Agra 16-May-07 25-Jun-07 24-Jun-09 Microbiology, Pathology Pathology: Onco Pathology, AIDS & Virology. NON NABH NON NABH 282002 FAX – 05622152516 E.Mail – [email protected] 6 Pankaj Scanning and Pathology 146/305, Shopping Arcade, Tel:0562305800,3057010, LUCKNOW Agra 13-May-05 28-Mar-14 27-Mar-16 Pathology and Radio Diagnosis Radio Diagnosis/ Imaging - CT Scan and MRI NON NABH NON NABH Research Centre Pvt Ltd Sadar Bazar, Agra-282001 7 Pushpanjali Hospital and Pushpanjali Palace, Delhi Mr.