Spatiotemporal Dynamics of Mediterranean Shallow Coastal Fish Communities Along a Gradient of Marine Protection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Digital Heritage
EuroMed2010 Digital Heritage 3rd International Conference dedicated on Digital Heritage Short Papers Before July, 1974… After July, 1974… The Katholikon of the Monastery of Antiphonitis in Kalogrea, Cyprus (16th century) DON’T MISS THE Hi-tech-STORY… 8th–13th of November 2010 Limassol, Cyprus Edited by M. Ioannides, D. Fellner, A. Georgopoulos, D. Hadjimitsis M. Ioannides, D. Fellner, A. Georgopoulos, D. Hadjimitsis (Eds) Marinos Ioannides Editor-in-Chief Elizabeth Jerem Managing Editor Elizabeth Jerem Copy Editor ARCHAEOLINGUA Cover Design Carol Usher, Gergely Hős Desktop editing and layout Front and Back Cover Images: Christ Antifonitis near Kalogrea in the Kyrenia district in Cyprus is another church, which was built and decorated with wall paintings in approximately 1200 AD. In the early 16th century, the north and south wall of the church proper and the northwestern squinch were decorated with wall paintings from the life of the Virgin, the Stem of Jesse, the Last Judgment and the standing saints, while the cupola was decorated with Christ Pantocrator, the Deesis-Supplication, the Apostles seated on thrones and the Prophets. Unfortunately, after the Turkish invasion in 1974 and the occupation of the area by the Turkish army, the systematic destruction and removal of the wall paintings from the monument began. The faces of Archangels Michael and Gabriel in the conch of the apse were barbarically destroyed, while the wall painting of the Birth of Christ was removed. These wall paintings are dated from the end of the 12th or early in the 13th century. The great wall paintings of the Stem of Jesse and the Last Judgment (early 16th century) were fragmented into small pieces and removed from the south and the north wall on which they were painted. -

The Blue Paradox: Preemptive Overfishing in Marine Reserves
PAPER The blue paradox: Preemptive overfishing in COLLOQUIUM marine reserves Grant R. McDermotta,1,2, Kyle C. Mengb,c,d,1,2, Gavin G. McDonaldb,e, and Christopher J. Costellob,c,d,e aDepartment of Economics, University of Oregon, Eugene, OR 97403; bBren School of Environmental Science & Management, University of California, Santa Barbara, CA 93106; cDepartment of Economics, University of California, Santa Barbara, CA 93106; dNational Bureau of Economic Research, Cambridge, MA 02138; and eMarine Science Institute, University of California, Santa Barbara, CA 93106 Edited by Jane Lubchenco, Oregon State University, Corvallis, OR, and approved July 25, 2018 (received for review March 9, 2018) Most large-scale conservation policies are anticipated or an- This line of reasoning suggests that the anticipation of a new nounced in advance. This risks the possibility of preemptive conservation policy may give rise to a set of incentives that is resource extraction before the conservation intervention goes distinct from—but possibly just as important as—the incentives into force. We use a high-resolution dataset of satellite-based fish- arising from the policy’s implementation. While this kind of pre- ing activity to show that anticipation of an impending no-take emptive behavior has been well-documented for landowners, gun marine reserve undermines the policy by triggering an unin- owners, and owners of natural resource extraction rights, it has tended race-to-fish. We study one of the world’s largest marine not been studied in the commons. For vast swaths of the ocean, reserves, the Phoenix Islands Protected Area (PIPA), and find that no single owner has exclusive rights and so must compete against fishers more than doubled their fishing effort once this area was others for extraction. -

The Mediterranean in Italian Baroque Theater
eScholarship California Italian Studies Title Bodies of Water: The Mediterranean in Italian Baroque Theater Permalink https://escholarship.org/uc/item/8dv7n1dk Journal California Italian Studies, 1(1) Author Snyder, Jon Publication Date 2010 DOI 10.5070/C311008855 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Bodies of Water: The Mediterranean in Italian Baroque Theater Jon R. Snyder The Mediterranean is not merely geography. [. .] The Mediterranean is not merely history. — Predrag Matvejevi ć 1 Così convertendo il suo remo in penna, il suo mare in inchiostro, e la sua trireme in un libro manuscritto, il feci scorrer per l’Egeo de’ letterati, accioché mi dicessero, s’egli era bastante per resistere agli assalti de’ pirati malevoli, e fatto sicuro alfine da mordaci incursioni, alle stampe il diedi. 2 Thus wrote Giovan Battista Andreini (1576-1654), arguably the leading Italian comic playwright of the seventeenth century, in the dedicatory preface to the revised edition of his early comedy entitled Lo schiavetto (The Little Slave). 3 In personifying the process through which the original script of the play was transformed into this published literary work, Andreini employs an extended metaphor that must have made ready sense to his contemporary Baroque readership. For the character whose name also serves as the title of the work is portrayed in these lines as an unwilling participant in a central institution of early modernity—one whose very existence depended upon Mediterranean voyages. The “little slave” is, without a doubt, a galley slave, although one who in Italy will exchange his “oar” for a “pen,” salt water for “ink,” and his ship for a manuscript. -

Marine Protected Areas (Mpas) in Management 1 of Coral Reefs
ISRS BRIEFING PAPER 1 MARINE PROTECTED AREAS (MPAS) IN MANAGEMENT 1 OF CORAL REEFS SYNOPSIS Marine protected areas (MPAs) may stop all extractive uses, protect particular species or locally prohibit specific kinds of fishing. These areas may be established for reasons of conservation, tourism or fisheries management. This briefing paper discusses the potential uses of MPAs, factors that have affected their success and the conditions under which they are likely to be effective. ¾ MPAs are often established as a conservation tool, allowing protection of species sensitive to fishing and thus preserving intact ecosystems, their processes and biodiversity and ultimately their resilience to perturbations. ¾ Increases in charismatic species such as large groupers in MPAs combined with the perception that the reefs there are relatively pristine mean that MPAs can play a significant role in tourism. ¾ By reducing fishing mortality, effective MPAs have positive effects locally on abundances, biomass, sizes and reproductive outputs of many exploitable site- attached reef species. ¾ Because high biomass of focal species is sought but this is quickly depleted and is slow to recover, poaching is a problem in most reef MPAs. ¾ Target-species ‘spillover’ into fishing areas is likely occurring close to the MPA boundaries and benefits will often be related to MPA size. Evidence for MPAs acting as a source of larval export remains weak. ¾ The science of MPAs is at an early stage of its development and MPAs will rarely suffice alone to address the main objectives of fisheries management; concomitant control of effort and other measures are needed to reduce fishery impacts, sustain yields or help stocks to recover. -

Grain Trade and the Genoese Dominating Minority in Sicily and Tabarka (16Th-18Th Centuries)
ORE Open Research Exeter TITLE Whatever the cost: Grain trade and the Genoese dominating minority in Sicily and Tabarka (16th-18th centuries) AUTHORS Iodice, A; Piccinno, L JOURNAL Business History DEPOSITED IN ORE 23 July 2021 This version available at http://hdl.handle.net/10871/126508 COPYRIGHT AND REUSE Open Research Exeter makes this work available in accordance with publisher policies. A NOTE ON VERSIONS The version presented here may differ from the published version. If citing, you are advised to consult the published version for pagination, volume/issue and date of publication Business History ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/fbsh20 Whatever the cost: Grain trade and the Genoese dominating minority in Sicily and Tabarka (16th-18th centuries) Antonio Iodice & Luisa Piccinno To cite this article: Antonio Iodice & Luisa Piccinno (2021): Whatever the cost: Grain trade and the Genoese dominating minority in Sicily and Tabarka (16th-18th centuries), Business History, DOI: 10.1080/00076791.2021.1924686 To link to this article: https://doi.org/10.1080/00076791.2021.1924686 © 2021 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group Published online: 31 May 2021. Submit your article to this journal Article views: 168 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=fbsh20 BUSINESS HISTORY https://doi.org/10.1080/00076791.2021.1924686 Whatever the cost: Grain trade and the Genoese dominating minority in Sicily and Tabarka (16th-18th centuries) Antonio Iodicea,b and Luisa Piccinnoa aDepartment of Economics and Business Studies, University of Genoa, Genoa, Italy; bDigital Humanities Lab, University of Exeter, Exeter, UK ABSTRACT KEYWORDS This work analyses the activities of Genoese merchant communities in Genoese merchants; the grain trade in western Mediterranean markets. -
Global Patterns in Marine Mammal Distributions
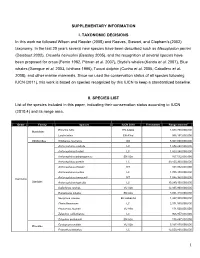
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

The Role of Marine Protected Areas in Sustaining Fisheries
The role of marine protected areas in sustaining fisheries Callum Roberts University of York, UK After World War II there was much optimism that fisheries could feed the World. But at the beginning of the 21st century, we are not so sure. Quota management of fisheries in the European Union has failed to deliver sustainability 100 80 60 40 20 Percentage of Quota Fish Stocks of Quota Percentage 0 1970 1975 1980 1985 1990 1995 2000 Year Healthy At risk In danger Data from ICES Cod decline in the Kattegat, North Sea Extinction is the ultimate in unsustainable fishing, whether or not the species of concern are targets of the fishery What is missing from fishery management? • Real provision for habitat protection and recovery • Precautionary targets • Resolute enforcement Objectives of marine reserves Maintaining ecosystem processes and services Conservation Sustaining fisheries Tree cover www.worldwildlife.org/oceans/pdfs/fishery_effects.pdf Spillover Reproduction & Dispersal Colonization & Growth Abundance Diversity What is the evidence that reserves work? Reserves all over the world show dramatic increases in spawning stocks Usually by at least 2-3 times in 5-10 years Long-term studies in New Zealand, Philippines, Florida and many other countries show strong responses to reserve protection Fish in reserves do live longer, grow larger and produce more eggs Egg production from protected fish stocks increases by much more than stock biomass Catches do increase Soufrière Marine Management Area, St. Lucia: Established 1995 35% of reef area closed -

Micro-Reserves, Consolidated Experience from the Valencian Community (Spain) by Emilio Laguna *
0055 Micro-reserves, consolidated experience from the Valencian Community (Spain) By Emilio Laguna * BASIC CHARACTERISTICS OF THE VALENCIAN COMMUNITY AND THE PLANT MICRORESERVE INITIATIVE In 1991, the author of this article proposed to the Generalitat Valenciana A new perspective: sites to be studied aswell as be protected - the autonomous government of the Valencian Community in Spain that The true starting point to build the plant micro-reserves (PMR) network a huge network of small protected sites should be created in order to was the EC's approval of a fund aid to the Generalitat Valenciana, during ensure the future study and monitoring of the rich endemic flora within the first call for proposals to the LIFE-Nature programme, in 1992. This the three Valencian provinces: Castellon, Valencia and Alicante. The was a project between 1993-97, later extended to 1999 that proposed the Valencian Community is one of the few European regions sharing more definition of a first set of 150 microsites - a kind of skeleton of the PMR than one Centre of Plant Diversity (see Davis & al., 1994), due to the geo- network, giving them a legal status as protected areas. This condition was graphical convergence of two major mountain systems in Spain: the achieved thanks to the Decree 217/1994 of the Valencian Government, Iberian-Javalambre mountains and the Betic Cordillera. This region has a which created the legal designation of 'Plant Micro-Reserve'. An initial very wide range in altitude (0-1832 m.a.s.l.), rainfall (from 200 to 1000 technical proposal showed that the Generalitat Valenciana could esta- mm/year) and mean annual temperatures (from 8 to 19ºC), as well as an blish a quite effective network designating only public sites managed by extreme variety of soil and rock types. -

Alicante2017 ING.Qxp 000-000 Portada Alicante ING 14/3/17 9:46 Página 1
000-000 Portada Alicante2017 ING.qxp_000-000 Portada Alicante ING 14/3/17 9:46 Página 1 Alicante “In my city, from the moment we are born, our eyes fill up with the blue from the sea.” Gabriel Miró. www.comunitatvalenciana.com ALICANTE 000-000 Portada Alicante2017 ING.qxp_000-000 Portada Alicante ING 14/3/17 9:46 Página 2 Alicante. 38º 20’ N 0º 28’ O www.comunitatvalenciana.com 001-033 Alicante ING 2017.qxp_001-033 Alicante ING 6/3/17 7:59 Página 1 001-033 Alicante ING 2017.qxp_001-033 Alicante ING 6/3/17 7:59 Página 2 Author: Pepe Serrano 001-033 Alicante ING 2017.qxp_001-033 Alicante ING 6/3/17 7:59 Página 3 Let yourself be seduced by a city of human dimensions 04 Alicante 05 A Shot of Light 08 A Sea that Sings 13 Des del matxo del castell... [from the top of the castle]… Advances and Vicissitudes 16 The Call of Tourism 17 Whispering Wind 20 Itinerary 1. In Proportion with Stone. The Monumental Route 22 Built Heritage 34 Itinerary 2. A City Within Reach. Charming Corners 36 ...s’abaniquen les palmeres [fanning palm trees] 42 Itinerary 3. Maritime Facade. A Stroll Looking out to Sea 47 Cosmopolitan Light 49 Multicolour Souk 54 Much more than Rice 58 A City Celebrating Tradition and Renewal 59 A la llum de Les fogueres...[By the light of the bonfires] 63 Pilgrim Light 66 Itinerary 4. Alicante Art. The Museum Route 74 Dazzled by Sport 78 Night-time Light 79 Alicante in Full Swing “The neighbourhood” 84 Projecting the Light. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Guidelines for Marine Protected Areas
Guidelines for Marine Protected Areas World Commission on Protected Areas (WCPA) Guidelines for Marine MPAs are needed in all parts of the world – but it is vital to get the support Protected Areas of local communities Edited and coordinated by Graeme Kelleher Adrian Phillips, Series Editor IUCN Protected Areas Programme IUCN Publications Services Unit Rue Mauverney 28 219c Huntingdon Road CH-1196 Gland, Switzerland Cambridge, CB3 0DL, UK Tel: + 41 22 999 00 01 Tel: + 44 1223 277894 Fax: + 41 22 999 00 15 Fax: + 44 1223 277175 E-mail: [email protected] E-mail: [email protected] Best Practice Protected Area Guidelines Series No. 3 IUCN The World Conservation Union The World Conservation Union CZM-Centre These Guidelines are designed to be used in association with other publications which cover relevant subjects in greater detail. In particular, users are encouraged to refer to the following: Case studies of MPAs and their Volume 8, No 2 of PARKS magazine (1998) contributions to fisheries Existing MPAs and priorities for A Global Representative System of Marine establishment and management Protected Areas, edited by Graeme Kelleher, Chris Bleakley and Sue Wells. Great Barrier Reef Marine Park Authority, The World Bank, and IUCN. 4 vols. 1995 Planning and managing MPAs Marine and Coastal Protected Areas: A Guide for Planners and Managers, edited by R.V. Salm and J.R. Clark. IUCN, 1984. Integrated ecosystem management The Contributions of Science to Integrated Coastal Management. GESAMP, 1996 Systems design of protected areas National System Planning for Protected Areas, by Adrian G. Davey. Best Practice Protected Area Guidelines Series No. -

Oregon Marine Reserves Ecological Monitoring Plan 2012
Oregon Marine Reserves Ecological Monitoring Plan 2012 Marine Resources Program Newport, Oregon Acknowledgments: Thank you to the Redfish Rocks and Otter Rock Marine Reserve Community Teams, biological working groups, local fishermen, divers and scientific experts for their time, hard work, and contribution to the development of the monitoring plan and to this document. Individual Acknowledgments: Otter Rock Community Team Biological Working Group: Jim Burke, Terry Thompson, Paul Erskine and Gary Wise Redfish Rocks Community Team Science Working Group: Jim Golden, Dick Vander Schaaf, Dave Lacey, Brianna Goodwin, Lyle Keeler Advisors: Mark Carr, Mark Hixon, Alan Shanks, Rick Starr, Brian Tissot and the members of the Ocean Policy and Advisory Council’s Science and Technical Advisory Committee. Contributors: Alix Laferriere, Cristen Don, David Fox, Keith Matteson, Mike Donnellan, Scott Groth, Annie Pollard. Cover photo: Scott Groth. Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-7701 x 227 www.dfw.state.or.us/MRP/ www.oregonocean.info/marinereserves Oregon Department of Fish and Wildlife Table of Contents I. Introduction ............................................................................................................................................. 1 A. Monitoring Plan Purpose..................................................................................................................... 1 B. Marine Reserves: Oregon’s Policy Guidance ....................................................................................