Rescueflow Sol F Inf 250 Ml
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiovascular Effect of 7.5% Sodium Chloride Dextran Infusion After
ORIGINAL ARTICLE Cardiovascular Effect of 7.5% Sodium Chloride–Dextran Infusion After Thermal Injury Joseph T. Murphy, MD; Jureta W. Horton, PhD; Gary F. Purdue, MD; John L. Hunt, MD Hypothesis: Clinical study can help determine the safety RL alone (mean ± SEM, 0.45 ± 0.32 vs 1.35 ± 0.35 µg/L and cardiovascular and systemic effects of an early infu- at 8 hours, 0.88 ± 0.55 vs 2.21 ± 0.35 µg/L at 12 hours). sion of 7.5% sodium chloride in 6% dextran-70 (hyper- While cardiac output increased proportionately be- tonic saline–dextran-70 [HSD]) given as an adjuvant to tween 4 and 24 hours in both groups (from 5.79 ± 0.8 to a standard resuscitation with lactated Ringer (RL) solu- 9.45 ± 1.1 L/min [mean ± SEM] for HSD vs from 5.4 ± 0.4 tion following severe thermal injury. to 9.46 ± 1.22 L/min for RL), filling pressure (central ve- nous pressure and pulmonary capillary wedge pressure) Design: Prospective clinical study. remained low for 12 hours after HSD infusion (P = .048). Total fluid requirements at 8 hours (2.76 ± 0.7 mL/kg per Setting: Intensive care unit of tertiary referral burn care each 1% TBSA burned [mean ± SEM] for HSD vs center. 2.67 ± 0.24 mL/kg per each 1% TBSA burned for RL) and 24 hours (6.11 ± 4.4 vs 6.76 ± 0.75 mL/kg per each 1% TBSA Patients: Eighteen patients with thermal injury over burned) were similar. Blood pressure remained un- more than 35% of the total body surface area (TBSA) changed, and serum sodium levels did not exceed 150 ± 2 (range, 36%-71%) were studied. -

Pediatric Hypovolemic Shock Michael J
Send Orders of Reprints at [email protected] 10 The Open Pediatric Medicine Journal, 2013, 7, (Suppl 1: M3) 10-15 Open Access Pediatric Hypovolemic Shock Michael J. Hobson1,2 and Ranjit S. Chima*,1,2 1Division of Critical Care Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA 2Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA Abstract: Hypovolemic shock is a common yet underappreciated insult which often accompanies illnesses afflicting children. Indeed, it is by far the most common type of shock in the pediatric age group worldwide. Early recognition and treatment of hypovolemic shock is paramount to reversing cellular hypoxia and ischemia before irreparable end-organ damage ensues. Keywords: Hypovolemic shock, dehydration, hemorrhage. INTRODUCTION for the administration of diluted juices or formula which may put the patient at risk for hyponatremia. Hypernatremia Hypovolemic shock is a common yet underappreciated results from an excessive loss of free water relative to insult which often accompanies illnesses afflicting children. sodium; the reverse is true in the case of hyponatremic Early recognition and treatment of shock is paramount to dehydration. The causes of dehydration and hypovolemic reversing cellular hypoxia and ischemia before irreparable shock in children are numerous (Table 2), but can be broadly end-organ damage ensues. Described over 150 years ago, defined by either decreased intake of fluid, excessive hypovolemic shock remains the most common etiology of gastrointestinal losses, excessive urinary losses, or shock affecting children today. Diarrheal illnesses resulting translocation of body fluid from the intravascular in dehydration account alone for approximately 30% of compartment. -

High Dosage of Dextran 70 Is Associated with Severe Bleeding in Patients Admitted to the Intensive Care Unit for Septic Shock
Dan Med J 59/11 November 2012 DANISH MEDICAL JOURNAL 1 High dosage of dextran 70 is associated with severe bleeding in patients admitted to the intensive care unit for septic shock Lisa Nebelin Hvidt & Anders Perner ABSTRACT tages of resuscitation with synthetic colloids versus crys- ORIGINAL INTRODUCTION: Synthetic colloids are frequently used in talloids remain heavily debated. ARTICLE fluid resuscitation of septic patients. Despite this, little is Serious adverse effects of colloids have been re- Department of known about the potential side effects including the risk ported including acute kidney injury (AKI) [4, 5], bleeding Intensive Care, of renal failure and bleeding. As practice has changed, we [6] and increased mortality [7]. The newly published 6S Rigshospitalet performed a before-and-after study of fluid resuscitation trial demonstrated an increased risk of death at 90 days and outcome in patients with septic shock. and an increase in the need for renal replacement ther- Dan Med J 2012;59(11):A4531 MATERIAL AND METHODS: We retrospectively assessed apy when HES 130/0.42 was used in severe sepsis [8]. all adult patients with septic shock admitted to a general Comparable results were observed in a study of HES intensive care unit (ICU) at a tertiary hospital in the years 200/0.5 [4]. 2006 and 2008. Data on patient characteristics, resuscita- Dextran 70 is a synthetic colloid of glucopolysac- tion fluids in the ICU and outcome were collected from charides which is still being used in the resuscitation of electronic databases and patient files. septic patients in Scandinavia, but to a lesser extent RESULTS: A total of 332 patients with septic shock were in- than other colloid solutions [9]. -

Review Small Volume Hypertonic Resuscitation of Circulatory Shock
CLINICS 60(2):159-172, 2005 REVIEW SMALL VOLUME HYPERTONIC RESUSCITATION OF CIRCULATORY SHOCK Mauricio Rocha-e-Silva and Luiz F. Poli de Figueiredo ROCHA-E-SILVA et al. Small volume hypertonic resuscitation of circulatory shock. CLINICS 60(2):159-172, 2005. Small volume hypertonic resuscitation is a relatively new conceptual approach to shock therapy. It was originally based on the idea that a relatively large blood volume expansion could be obtained by administering a relatively small volume of fluid, taking advantage of osmosis. It was soon realized that the physiological vasodilator property of hypertonicity was a useful byproduct of small volume resuscitation in that it induced reperfusion of previously ischemic territories, even though such an effect encroached upon the malefic effects of the ischemia-reperfusion process. Subsequent research disclosed a number of previously unsuspected properties of hypertonic resuscitation, amongst them the correction of endothelial and red cell edema with significant consequences in terms of capillary blood flow. A whole set of actions of hypertonicity upon the immune system are being gradually uncovered, but the full implication of these observations with regard to the clinical scenario are still under study. Small volume resuscitation for shock is in current clinical use in some parts of the world, in spite of objections raised concerning its safety under conditions of uncontrolled bleeding. These objections stem mainly from experimental studies, but there are few signs that they may be of real clinical significance. This review attempts to cover the earlier and the more recent developments in this field. KEYWORDS: Shock therapy. Hemorrhage. Hypertonic saline. -

Calcium Alginate/Dextran 70 1059
Calcium Alginate/Dextran 70 1059 which the linkages between glucose units are almost exclusively renal disease with oliguria; should anuria or oliguria occur dur- Hung.: Rheomacrodex; Israel: Rheomacrodex; Ital.: Eudextran; Plander α-1,6. Its weight average molecular weight is about 1000. ing treatment dextran 40 should be withdrawn. Dehydration R; Solplex 40†; Mex.: Rheomacrodex; Norw.: Rheomacrodex; Philipp.: LM Dextran; Port.: Neodextril 40; Rus.: Rheomacrodex (Реомакродекс); A white to off-white, hygroscopic powder. Very soluble in water; should preferably be corrected before giving dextran 40. Dextran Rheopolydex (Реополидекс); Rheopolyglukin with Glucose sparingly soluble in alcohol. pH of a 15% solution in water is be- 40 can cause capillary oozing of wound surfaces. (Реополиглюкин С Глюкозой); S.Afr.: Rheomacrodex; Spain: Rheomac- tween 4.5 and 7.0. Store at a temperature between 4° and 30°. Effects on the kidneys. Acute renal failure has been associat- rodex; Swed.: Perfadex; Rheomacrodex; Switz.: Rheomacrodex†; Thai.: 1-4 1 Onkovertin; Turk.: Rheomacrodex; UK: Gentran 40; USA: Gentran 40; Profile ed with dextran 40 and less frequently with dextran 70. The Rheomacrodex. Dextran 1 is used to prevent severe anaphylactic reactions to in- mechanism of the effect is unclear but suggestions include an in- Multi-ingredient: Indon.: Otsutran; Port.: Bas-Dextrano; Rus.: Rhe- fusions of dextran. It is reported to occupy the binding sites of crease in plasma oncotic pressure that decreases filtration pres- ogluman (Реоглюман). dextran-reactive antibodies and so prevent the formation of large sure in the glomerulus and hence decreases glomerular filtration immune complexes with higher molecular weight dextrans. rate,2 obstruction within the tubules,2,4 or a direct toxic effect on renal cells.4 Plasmapheresis has been used successfully to re- Dextran 1 is given in usual doses of 20 mL of a solution contain- 2-4 ing 150 mg/mL by intravenous injection about 1 to 2 minutes move dextran from the circulation. -

The Selection and Use of Essential Medicines
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the World Health Organization WHO Technical Report Series 933 THE SELECTION AND USE OF ESSENTIAL MEDICINES Report of the WHO Expert Committee, 2005 (including the 14th Model List of Essential Medicines) World Health Organization Geneva 2006 i WHO Library Cataloguing-in-Publication Data WHO Expert Committee on the Selection and Use of Essential Medicines (14th : 2005: Geneva, Switzerland) The selection and use of essential medicines : report of the WHO Expert Committee, 2005 : (including the 14th model list of essential medicines). (WHO technical report series ; 933) 1.Essential drugs — standards 2.Formularies — standards 3.Drug information services — organization and administration 4.Drug utilization 5. Pharmaceutical preparations — classification 6.Guidelines I.Title II.Title: 14th model list of essential medicines III.Series. ISBN 92 4 120933 X (LC/NLM classification: QV 55) ISSN 0512-3054 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publica- tions — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Are Colloid Solutions Essential for the Treatment of Pediatric Trauma Or Burn Patients?
Review for the Expert Committee on the Selection and Use of Essential Medicines Are colloid solutions essential for the treatment of pediatric trauma or burn patients? Christina Huwer Volunteer Department of Violence and Injury Prevention and Disability World Health Organization Geneva, Switzerland November 2012 Content Summary………………………………………………………………………………………………………………………..2 Objective………………………………………………………………………………………………………………………..3 Background…………………………………………………………………………………………………………………….3 Search Methods……………………………………………………………………………………………………………..4 Results…………………………………………………………………………………………………………………………….5 Efficacy…………………………………………………………………………………………………………………………...7 Safety……………………………………………………………………………………………………………………………..9 Costs……………………………………………………………………………………………………………………………….9 Conclusion…………………………………………………………………………………………….………………………10 Other Indications………………………………………………………………………………………………………….10 References……………………………………………………………………………………………………………………11 Appendix………………………………………………………………………………………………………………………13 1 Summary Colloid solutions are widely used for fluid resuscitation including children, although there is an ongoing controversy concerning their actual use in children. To support the Expert Committee on the Selection and Use of Essential Medicines in their decision whether to include a colloid solution in the Essential Medicines List for Children this review of the literature was conducted. No high-level evidence studies could be identified to answer the question. From the available evidence it appears that colloid solutions do not have a -
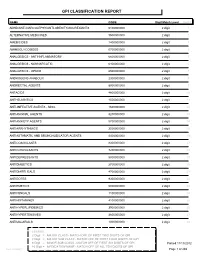
Gpi Drug Classification Report
GPI CLASSIFICATION REPORT NAME CODE Digit Match Level Count ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS 6100000000 2 digit 1 ALTERNATIVE MEDICINES 9500000000 2 digit 2 AMEBICIDES 1400000000 2 digit 3 AMINOGLYCOSIDES 0700000000 2 digit 4 ANALGESICS - ANTI-INFLAMMATORY 6600000000 2 digit 5 ANALGESICS - NONNARCOTIC 6400000000 2 digit 6 ANALGESICS - OPIOID 6500000000 2 digit 7 ANDROGENS-ANABOLIC 2300000000 2 digit 8 ANORECTAL AGENTS 8900000000 2 digit 9 ANTACIDS 4800000000 2 digit 10 ANTHELMINTICS 1500000000 2 digit 11 ANTI-INFECTIVE AGENTS - MISC. 1600000000 2 digit 12 ANTIANGINAL AGENTS 3200000000 2 digit 13 ANTIANXIETY AGENTS 5700000000 2 digit 14 ANTIARRHYTHMICS 3500000000 2 digit 15 ANTIASTHMATIC AND BRONCHODILATOR AGENTS 4400000000 2 digit 16 ANTICOAGULANTS 8300000000 2 digit 17 ANTICONVULSANTS 7200000000 2 digit 18 ANTIDEPRESSANTS 5800000000 2 digit 19 ANTIDIABETICS 2700000000 2 digit 20 ANTIDIARRHEALS 4700000000 2 digit 21 ANTIDOTES 9300000000 2 digit 22 ANTIEMETICS 5000000000 2 digit 23 ANTIFUNGALS 1100000000 2 digit 24 ANTIHISTAMINES 4100000000 2 digit 25 ANTIHYPERLIPIDEMICS 3900000000 2 digit 26 ANTIHYPERTENSIVES 3600000000 2 digit 27 ANTIMALARIALS 1300000000 2 digit 28 LEGEND: 2 Digit = MAJOR CLASS - MATCH OFF OF FIRST TWO DIGITS OF GPI 4 Digit = MAJOR SUB CLASS - MATCH OFF OF FIRST FOUR DIGITS OF GPI 6 Digit = MINOR SUB CLASS - MATCH OFF OF FIRST SIX DIGITS OF GPI Printed:11/13/2012 10 Digit = MEDICATION NAME - MATCH OFF OF ALL TEN DIGITS OF GPI Lee Cooper Page 1 of 265 NAME CODE Digit Match Level Count ANTIMYASTHENIC AGENTS -

Intravenous Volume Replacement: Which Fluid and Why?
A,vchives ofDisease in Childhood 1992; 67: 649-653 649 ;URRENT TOPIC Arch Dis Child: first published as 10.1136/adc.67.5.649 on 1 May 1992. Downloaded from Intravenous volume replacement: which fluid and why? Lucinda Huskisson Fluids available for intravenous volume replace- The interstitial space fills more readily after ment may be either crystalloid or colloid. The crystalloid resuscitation. Because of this, the fundamental differences between these fluids volume of fluid required is two to three times are their effects on the Starling equation (table greater than when using colloids, resulting in an 1) which describes fluid flux between the increased risk of tissue oedema. Sponsors of the intravascular and interstitial spaces. Starling crystalloid school maintain that this is not stated that the rate of fluid movement into or harmful despite the fact that tissue oedema has out of a capillary is related to the net hydrostatic been associated with tissue hypoxia and has pressure minus the net colloid osmotic pressure.1 been implicated in delayed healing of bowel The Starling equation has been modified to anastomoses." Despite the increased volumes include coefficients which represent the per- required, crystalloid resuscitation is cheaper meability of the capillary membrane to small than the colloid equivalent (table 2). solutes (Kfc) and its ability to prevent large Velanovich analysed the mortality data from a molecules such as plasma proteins from crossing number of clinical trials and concluded that it (oc).' Colloids may be used to replenish the after trauma, or in instances when the capillaries oncotic strength of the blood, thereby enhanc- are likely to have increased permeability, resus- ing its water retaining capacity. -

Medialjurnal
MEDiaL URNAL J Adhemar Monteiro Pacheco Jr., Raul Sergio Martins Coimbra, Uwe Kreimeier, Lorenz Frey, Konrad Messmer Hypertonic volume therapy: feasibility in the prevention and treatment of multiple organ failure and sepsis Department of Surgery, Santa Casa School of Medicine, Institute for Anesthesiology, University of Munich, Germany Small-volume resuscitation by means of bolus infusion of hypertonic saline solutions was first applied for the primary treatment of severe hemorrhagic and traumatic shock and promptly restored central hemodynamics and regional organ blood flow. Mechanisms of action are diverse - i. maintenance of high cardiac output (direct myocardial stimulation; increase in intravascular volume); ii. maintenance of peripheral arterial vasodilation (effect of hyperosmolality; plasma volume effect) and iii. reduction of tissue edema (shifting of tissue water along the osmotic gradient). These mechanisms promote the restoration of the severely impaired microcircu- lation frequently seen also in sepsis. Hypertonic volume therapy has been the object of several experimental studies of acute hyperdynamic endotoxemia, however, a greater number of clinical studies have to be developed for the better understanding of the positive, and perhaps hazardous, effects of small-volume resuscitation in sepsis and multiple organ failure. The aim of this paper is to review the concepts involving such solutions, and their potential use in treatment of profound hypovolemia and microcirculatory deterioration associated with sepsis and endotoxic -

The Effectiveness of Prehospital Hypertonic Saline for Hypotensive Trauma Patients: a Systematic Review and Meta-Analysis I
Blanchard et al. BMC Emergency Medicine (2017) 17:35 DOI 10.1186/s12873-017-0146-1 RESEARCH ARTICLE Open Access The effectiveness of prehospital hypertonic saline for hypotensive trauma patients: a systematic review and meta-analysis I. E. Blanchard1,2,3*, A. Ahmad3, K. L. Tang4, P. E. Ronksley3, D. Lorenzetti3, G. Lazarenko1, E. S. Lang5, C. J. Doig2 and H. T. Stelfox2,3,4,6 Abstract Background: The optimal prehospital fluid for the treatment of hypotension is unknown. Hypertonic fluids may increase circulatory volume and mute the pro-inflammatory response of the body to injury and illness. The purpose of this systematic review is to determine whether in patients presenting with hypotension in the prehospital setting (population), the administration of hypertonic saline (intervention), compared to an isotonic fluid (control), improves survival to hospital discharge (outcome). Methods: Searches were conducted in Medline, Embase, CINAHL, and CENTRAL from the date of database inception to November, 2016, and included all languages. Two reviewers independently selected randomized control trials of hypotensive human participants administered hypertonic saline in the prehospital setting. The comparison was isotonic fluid, which included normal saline, and near isotonic fluids such as Ringer’s Lactate. Assessment of study quality was done using the Cochrane Collaborations’ risk of bias tool and a fixed effect meta-analysis was conducted to determine the pooled relative risk of survival to hospital discharge. Secondary outcomes were reported for fluid requirements, multi-organ failure, adverse events, length of hospital stay, long term survival and disability. Results: Of the 1160 non-duplicate citations screened, thirty-eight articles underwent full-text review, and five trials were included in the systematic review. -

Hypertonic Saline Dextran – the Fluid of Choice in the Resuscitation of Haemorrhagic Shock?
J R Army Med Corps 2003; 149: 110-120 J R Army Med Corps: first published as 10.1136/jramc-149-02-03 on 1 June 2003. Downloaded from Hypertonic Saline Dextran – The Fluid Of Choice In The Resuscitation Of Haemorrhagic Shock? W Sapsford Introduction compartment. Since the extracellular space The crystalloid – colloid debate regarding is 4-5 times larger than the plasma volume, the most effective intravenous fluid for only 10-20% of infused crystalloid remains resuscitation from haemorrhagic shock has in the circulation, requiring at least three been raging over the past 60 years, and still units of crystalloid to replace each unit of continues without a satisfactory resolution shed blood – the “3:1 rule” (2,5). The two (1). Crystalloid solutions are essentially major disadvantages of colloids are cost and isotonic salt solutions (270-310 mOsm/L) their potential complications (Table 1). and have been in use since the 1800s but While blood and blood products have their use became widespread during World remained the mainstays of massive War I. Normal saline or Ringer’s lactate transfusions, they are only available in the solutions currently predominate over all hospital environment and there is always the other fluids for intravenous volume support. risk of infection and immunological Colloids, on the other hand, are large reactions. Blood substitutes, such as haem- macromolecules that remain in the oglobin solutions, liposome encapsulated circulation and exert a colloid osmotic or haemoglobins and perfluorocarbons are still oncotic pressure due to their molecular in development and remain some years away weight. Their size also determines how long from routine use.