A Lagrangian Model of Air-Mass Photochemistry and Mixing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effects of the Atmosphere and Weather on Radio Astronomy Observations
The Effects of the Atmosphere and Weather on Radio Astronomy Observations Ronald J Maddalena July 2011 The Influence of the Atmosphere and Weather at cm- and mm-wavelengths Opacity Cloud Cover Calibration Continuum performance System performance – Tsys Calibration Observing techniques Winds Hardware design Pointing Refraction Safety Pointing Telescope Scheduling Air Mass Proportion of proposals Calibration that should be accepted Interferometer & VLB phase Telescope productivity errors Aperture phase errors Structure of the Lower Atmosphere Refraction Refraction Index of Refraction is weather dependent: .3 73×105 ⋅ P (mBar) 6 77 6. ⋅ PTotal (mBar) H 2O (n0 − )1 ⋅10 ≈ + +... T(C) + 273.15 ()T(C) + 273.15 2 P (mBar) ≈ 6.112⋅ea H 2O .7 62⋅TDewPt (C) where a ≈ 243.12 +TDewPt (C) Froome & Essen, 1969 http://cires.colorado.edu/~voemel/vp.html Guide to Meteorological Instruments and Methods of Observation (CIMO Guide) (WMO, 2008) Refraction For plane-parallel approximation: n0 • Cos(Elev Obs )=Cos(Elev True ) R = Elev Obs – Elev True = (n 0-1) • Cot(Elev Obs ) Good to above 30 ° only For spherical Earth: n 0 dn() h Elev− Elev = a ⋅ n ⋅cos( Elev ) ⋅ Obs True0 Obs 2 2 2 2 2 ∫1 nh()(⋅ ah + )() ⋅ nh − an ⋅0 ⋅ cos( Elev Obs ) a = Earth radius; h = distance above sea level of atmospheric layer, n(h) = index of refraction at height h; n 0 = ground-level index of refraction See, for example, Smart, “Spherical Astronomy” Refraction Important for good pointing when combining: large aperture telescopes at high frequencies at low elevations (i.e., the GBT) Every observatory handles refraction differently. Offset pointing helps eliminates refraction errors Since n(h) isn’t usually known, most (all?) observatories use some simplifying model. -

Air Mass Effect on the Performance of Organic Solar Cells
Available online at www.sciencedirect.com ScienceDirect Energy Procedia 36 ( 2013 ) 714 – 721 TerraGreen 13 International Conference 2013 - Advancements in Renewable Energy and Clean Environment Air mass effect on the performance of organic solar cells A. Guechi1*, M. Chegaar2 and M. Aillerie3,4, # 1Institute of Optics and Precision Mechanics, Ferhat Abbas University, 19000, Setif, Algeria 2L.O.C, Department of Physics, Faculty of Sciences, Ferhat Abbas University, 19000, Setif, Algeria Email : [email protected], [email protected] 3Lorraine University, LMOPS-EA 4423, 57070 Metz, France 4Supelec, LMOPS, 57070 Metz, France #Email: [email protected] Abstract The objective of this study is to evaluate the effect of variations in global and diffuse solar spectral distribution due to the variation of air mass on the performance of two types of solar cells, DPB (etraphenyl–dibenzo–periflanthene) and CuPc (Copper-Phthalocyanine) using the spectral irradiance model for clear skies, SMARTS2, over typical rural environment in Setif. Air mass can reduce the sunlight reaching a solar cell and thereby cause a reduction in the electrical current, fill factor, open circuit voltage and efficiency. The results indicate that this atmospheric parameter causes different effects on the electrical current produced by DPB and CuPc solar cells. In addition, air mass reduces the current of the DPB and CuPc cells by 82.34% and 83.07 % respectively under global radiation. However these reductions are 37.85 % and 38.06%, for DPB and CuPc cells respectively under diffuse solar radiation. The efficiency decreases with increasing air mass for both DPB and CuPc solar cells. © 20132013 The The Authors. -

Nighttime Photochemical Model and Night Airglow on Venus
Planetary and Space Science 85 (2013) 78–88 Contents lists available at ScienceDirect Planetary and Space Science journal homepage: www.elsevier.com/locate/pss Nighttime photochemical model and night airglow on Venus Vladimir A. Krasnopolsky n a Department of Physics, Catholic University of America, Washington, DC 20064, USA b Moscow Institute of Physics and Technology, Dolgoprudnyy 141700 Russia article info abstract Article history: The photochemical model for the Venus nighttime atmosphere and night airglow (Krasnopolsky, 2010, Received 23 December 2012 Icarus 207, 17–27) has been revised to account for the SPICAV detection of the nighttime ozone layer and Received in revised form more detailed spectroscopy and morphology of the OH nightglow. Nighttime chemistry on Venus is 28 April 2013 induced by fluxes of O, N, H, and Cl with mean hemispheric values of 3 Â 1012,1.2Â 109,1010, and Accepted 31 May 2013 − − 1010 cm 2 s 1, respectively. These fluxes are proportional to column abundances of these species in the Available online 18 June 2013 daytime atmosphere above 90 km, and this favors their validity. The model includes 86 reactions of 29 Keywords: species. The calculated abundances of Cl2, ClO, and ClNO3 exceed a ppb level at 80–90 km, and Venus perspectives of their detection are briefly discussed. Properties of the ozone layer in the model agree Photochemistry with those observed by SPICAV. An alternative model without the flux of Cl agrees with the observed O Night airglow 3 peak altitude and density but predicts an increase of ozone to 4 Â 108 cm−3 at 80 km. -

Ionospheric Reflections and Weather Forecasting for Eastern China*
Ionospheric Reflections and Weather Forecasting for Eastern China* REV. E. GHERZI, S.J. Director for Meteorology and Seismology, Zi Ka Wei Observatory, Shanghai, China HE FOLLOWING LINES describe some Another detail of our technique was that T very interesting and practical results we reduced to 20 watts or less the power obtained at the Zi Ka Wei Observa- radiated by our Hertz aerial, in order to get tory during the past five years, by means of reflections only from a well-ionized layer. the usual ionospheric radio soundings which This frequency of 6000 kc was used con- give the heights of the well-known E and F stantly during all these five years of research, layers. As early as 1936, Martin and Pulley and as we had at our disposal a file of (of the Radio Research Board, Melbourne) synoptic maps from our weather service, we reported on a "Correlation of conditions in quickly noticed a very interesting coincidence the ionosphere with barometric pressure at between the presence of an E or an F, or an the ground.,M We thought this idea very F2 echo, and the air mass which was "domi- interesting and started research along that nating the weather"! Namely, we found line. We wanted to find out if the motions that: (1) every time the Pacific trade-wind of the different air masses which produce air mass which was causing the weather, we the weather of our regions could be corre- had the E-layer reflection; (2) every time lated with the results obtained by the usual the Siberian air mass was dominating the ionospheric sounding technique. -

PHAK Chapter 12 Weather Theory
Chapter 12 Weather Theory Introduction Weather is an important factor that influences aircraft performance and flying safety. It is the state of the atmosphere at a given time and place with respect to variables, such as temperature (heat or cold), moisture (wetness or dryness), wind velocity (calm or storm), visibility (clearness or cloudiness), and barometric pressure (high or low). The term “weather” can also apply to adverse or destructive atmospheric conditions, such as high winds. This chapter explains basic weather theory and offers pilots background knowledge of weather principles. It is designed to help them gain a good understanding of how weather affects daily flying activities. Understanding the theories behind weather helps a pilot make sound weather decisions based on the reports and forecasts obtained from a Flight Service Station (FSS) weather specialist and other aviation weather services. Be it a local flight or a long cross-country flight, decisions based on weather can dramatically affect the safety of the flight. 12-1 Atmosphere The atmosphere is a blanket of air made up of a mixture of 1% gases that surrounds the Earth and reaches almost 350 miles from the surface of the Earth. This mixture is in constant motion. If the atmosphere were visible, it might look like 2211%% an ocean with swirls and eddies, rising and falling air, and Oxygen waves that travel for great distances. Life on Earth is supported by the atmosphere, solar energy, 77 and the planet’s magnetic fields. The atmosphere absorbs 88%% energy from the sun, recycles water and other chemicals, and Nitrogen works with the electrical and magnetic forces to provide a moderate climate. -

Atmosphere Aloft
Project ATMOSPHERE This guide is one of a series produced by Project ATMOSPHERE, an initiative of the American Meteorological Society. Project ATMOSPHERE has created and trained a network of resource agents who provide nationwide leadership in precollege atmospheric environment education. To support these agents in their teacher training, Project ATMOSPHERE develops and produces teacher’s guides and other educational materials. For further information, and additional background on the American Meteorological Society’s Education Program, please contact: American Meteorological Society Education Program 1200 New York Ave., NW, Ste. 500 Washington, DC 20005-3928 www.ametsoc.org/amsedu This material is based upon work initially supported by the National Science Foundation under Grant No. TPE-9340055. Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the National Science Foundation. © 2012 American Meteorological Society (Permission is hereby granted for the reproduction of materials contained in this publication for non-commercial use in schools on the condition their source is acknowledged.) 2 Foreword This guide has been prepared to introduce fundamental understandings about the guide topic. This guide is organized as follows: Introduction This is a narrative summary of background information to introduce the topic. Basic Understandings Basic understandings are statements of principles, concepts, and information. The basic understandings represent material to be mastered by the learner, and can be especially helpful in devising learning activities in writing learning objectives and test items. They are numbered so they can be keyed with activities, objectives and test items. Activities These are related investigations. -

Transfer of Diffuse Astronomical Light and Airglow in Scattering Earth Atmosphere
Earth Planets Space, 50, 487–491, 1998 Transfer of diffuse astronomical light and airglow in scattering Earth atmosphere S. S. Hong1,S.M.Kwon2, Y.-S. Park3, and C. Park1 1Department of Astronomy, Seoul National University, Seoul, Korea 2Department of Science Education, Kangwon National University, Korea 3Korea Astronomy Observatory, Yusong-Ku, Daejon, Korea (Received October 7, 1997; Revised February 24, 1998; Accepted February 27, 1998) To understand an observed distribution of atmospheric diffuse light (ADL) over an entire meridian, we have solved rigorously, with the quasi-diffusion method, the problem of radiative transfer in an anisotropically scattering spherical atmosphere of the earth. In addition to the integrated starlight and the zodiacal light we placed a narrow layer of airglow emission on top of the scattering earth atmosphere. The calculated distribution of the ADL brightness over zenith distance shows good agreement with the observed one. The agreement can be utilized in deriving the zodiacal light brightness at small solar elongations from the night sky brightness observed at large zenith distances. 1. Introduction ST◦(α, δ) exp[−τ(ζ)]. Here, τ(ζ) is the extinction optical One of the most difficult tasks in reducing the night sky ob- depth over the atmospheric path at ζ . servations to the zodiacal light is to remove the atmosphere- One may not use the same exponential factor to remove related diffuse light. Since air molecules and aerosols divert IS(α, δ; ζ), because it is an extended source. Formally the IS light from off the telescope axis into the telescope beam via is resulted from a “convolution” of the brightness IS◦(α, δ) multiple scattering, the brightness observed at a sky point before entering the atmosphere with a “transfer” function has contributions from not only the point itself but also the Tatm( α, δ; ζ)of the earth atmosphere: off-axis region. -

A New Approach to the Real-Time Assessment of the Clear-Sky Direct Normal Irradiance Julien Nou, Rémi Chauvin, Stéphane Thil, Stéphane Grieu
A new approach to the real-time assessment of the clear-sky direct normal irradiance Julien Nou, Rémi Chauvin, Stéphane Thil, Stéphane Grieu To cite this version: Julien Nou, Rémi Chauvin, Stéphane Thil, Stéphane Grieu. A new approach to the real-time assess- ment of the clear-sky direct normal irradiance. Applied Mathematical Modelling, Elsevier, 2016, 40 (15-16), pp.7245-7264. 10.1016/j.apm.2016.03.022. hal-01345997 HAL Id: hal-01345997 https://hal.archives-ouvertes.fr/hal-01345997 Submitted on 18 Jul 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A new approach to the real-time assessment of the clear-sky direct normal irradiance a a a,b a,b, Julien Nou ,Remi´ Chauvin , Stephane´ Thil , Stephane´ Grieu ∗ aPROMES-CNRS (UPR 8521), Rambla de la thermodynamique, Tecnosud, 66100 Perpignan, France bUniversit´ede Perpignan Via Domitia, 52 Avenue Paul Alduy, 66860 Perpignan, France Abstract In a context of sustainable development, interest for concentrating solar power and concentrating photovoltaic technologies is growing rapidly. One of the most challenging topics is to improve solar resource assessment and forecasting in order to optimize power plant operation. -
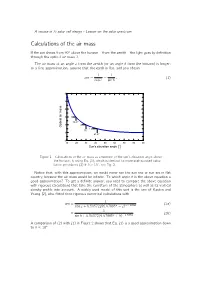
Calculations of the Air Mass
A course in Si solar cell design - Lesson on the solar spectrum Calculations of the air mass If the sun shines from 90± above the horizon { from the zenith { the light goes by de¯nition through the optical air mass 1. The air mass at an angle z from the zenith (or an angle h from the horizon) is longer: to a ¯rst approximation, assume that the earth is flat, and you obtain 1 1 am = = ; (1) cos z sin h 6 5 4 3 3 19.47° 2 2 Optical air mass 1.5 30° 1 41.81° 0 10 20 30 40 50 60 70 80 90 Sun's elevation angle [°] Figure 1 Calculations of the air mass as a function of the sun's elevation angle above the horizon, h, using Eq. (1), which is identical to more sophisticated calcu- lation procedures (2) if h > 10±, see Fig. 2. Notice that, with this approximation, we would never see the sun rise or sun set in flat country, because the air mass would be in¯nite. To which angle h is the above equation a good approximation? To get a de¯nite answer, you need to compare the above equation with rigorous calculations that take the curvature of the atmosphere as well as its vertical density pro¯le into account. A widely used model of this sort is the one of Kasten and Young [2], who ¯tted their rigorous numerical calculations with 1 am = (2a) cos z + 0:50572(96:07995± ¡ z)¡1:6364 1 = (2b) sin h + 0:50572(6:07995± + h)¡1:6364 A comparison of (2) with (1) in Figure 2 shows that Eq. -

Estimation of Hourly, Daily and Monthly Global Solar Radiation on Inclined Surfaces: Models Re-Visited
energies Review Estimation of Hourly, Daily and Monthly Global Solar Radiation on Inclined Surfaces: Models Re-Visited Seyed Abbas Mousavi Maleki 1,2,*, H. Hizam 1,2 and Chandima Gomes 1 1 Department of Electrical and Electronic Engineering, Universiti Putra Malaysia, Serdang, 43400 Selangor, Malaysia; [email protected] (H.H.); [email protected] (C.G.) 2 Centre of Advanced Power and Energy Research (CAPER), Universiti Putra Malaysia, 43400 Selangor, Malaysia * Correspondence: [email protected]; Tel.: +60-122-221-336 Academic Editor: Tatiana Morosuk Received: 20 September 2016; Accepted: 23 December 2016; Published: 22 January 2017 Abstract: Global solar radiation is generally measured on a horizontal surface, whereas the maximum amount of incident solar radiation is measured on an inclined surface. Over the last decade, a number of models were proposed for predicting solar radiation on inclined surfaces. These models have various scopes; applicability to specific surfaces, the requirement for special measuring equipment, or limitations in scope. To find the most suitable model for a given location the hourly outputs predicted by available models are compared with the field measurements of the given location. The main objective of this study is to review on the estimation of the most accurate model or models for estimating solar radiation components for a selected location, by testing various models available in the literature. To increase the amount of incident solar radiation on photovoltaic (PV) panels, the PV panels are mounted on tilted surfaces. This article also provides an up-to-date status of different optimum tilt angles that have been determined in various countries. -
![Arxiv:2107.02876V1 [Astro-Ph.IM] 6 Jul 2021 Technicians, and City Inspectors Were Done with Their Work, the System Went Live on 8 June](https://docslib.b-cdn.net/cover/4428/arxiv-2107-02876v1-astro-ph-im-6-jul-2021-technicians-and-city-inspectors-were-done-with-their-work-the-system-went-live-on-8-june-2854428.webp)
Arxiv:2107.02876V1 [Astro-Ph.IM] 6 Jul 2021 Technicians, and City Inspectors Were Done with Their Work, the System Went Live on 8 June
Including Atmospheric Extinction in a Performance Evaluation of a Fixed Grid of Solar Panels Kevin Krisciunas1;2 ABSTRACT Solar power is being used to satisfy a higher and higher percentage of our energy needs. We must be able to evaluate the performance of the hardware. Here we present an analysis of the performance of a fixed grid of 13 solar panels installed in the spring of 2021. We confirm that the power output is linearly proportional to the cosine of the angle of the incidence of sunlight with respect to the vector perpendicular to the panels. However, in order to confirm this, we need to consider the dimming effect of the Earth's atmosphere on the intensity of the Sun's light, which is to say we need to rely on astronomical photometric methods. We find a weighted mean value of 0.168 ± 0.007 mag/airmass for the extinction term, which corresponds to the sea level value at a wavelength of about 0.76 microns. As the efficiency of the solar panels is expected to degrade slowly over time, the data presented here provide a baseline for comparison to the future performance of the system. Subject headings: photometry - techniques 1. Introduction On 26 April 2021 Texas Green Energy installed two sets of solar panels3 on my house in College Station, Texas (latitude +30.56◦, longitude W 96.27◦, elevation 103 m). Six panels are mounted on the upper roof, and seven panels on the lower roof. After engineers, arXiv:2107.02876v1 [astro-ph.IM] 6 Jul 2021 technicians, and city inspectors were done with their work, the system went live on 8 June. -

Chapter 4: Principles of Flight
Chapter 4 Principles of Flight Introduction This chapter examines the fundamental physical laws governing the forces acting on an aircraft in flight, and what effect these natural laws and forces have on the performance characteristics of aircraft. To control an aircraft, be it an airplane, helicopter, glider, or balloon, the pilot must understand the principles involved and learn to use or counteract these natural forces. Structure of the Atmosphere The atmosphere is an envelope of air that surrounds the Earth and rests upon its surface. It is as much a part of the Earth as the seas or the land, but air differs from land and water as it is a mixture of gases. It has mass, weight, and indefinite shape. The atmosphere is composed of 78 percent nitrogen, 21 percent oxygen, and 1 percent other gases, such as argon or helium. Some of these elements are heavier than others. The heavier elements, such as oxygen, settle to the surface of the Earth, while the lighter elements are lifted up to the region of higher altitude. Most of the atmosphere’s oxygen is contained below 35,000 feet altitude. 4-1 Air is a Fluid the viscosity of air. However, since air is a fluid and has When most people hear the word “fluid,” they usually think viscosity properties, it resists flow around any object to of liquid. However, gasses, like air, are also fluids. Fluids some extent. take on the shape of their containers. Fluids generally do not resist deformation when even the smallest stress is applied, Friction or they resist it only slightly.