Rapid and Repeated Origin of Insular Gigantism and Dwarfism in Australian Tiger Snakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sir Joseph Banks Group
Sir Joseph Banks Group The Sir Joseph bank Group comprising about 20 islands, islets and rocks is less than 25 miles from Port Lincoln and therefore an easy day sail. The trip is made more complicated than a glance at the chart might suggest because of the presence of many fish farms extending well to the east and north of Boston Bay making the trip somewhat of an ‘obstacle course’. Although these island are low-lying and do not have the soaring cliffs and rugged coastline of the more southerly Thistle and Wedge Islands, they do have a charm and beauty of their own, and are definitely worth visiting. There are numerous reefs, isolated rocks and shallow patches within the Group, so careful navigation and attention to the tide are required. All the islands are part of the Sir Joseph Banks Marine Park which means some activities are restricted in designated areas. Current information can be found on the National Parks and Wildlife Service website https://www.environment.sa.gov.au/marineparks/find-a-park/eyre-peninsula/sir-joseph-banks Homestead Bay Shelter from NE – E – SE Indicative Anchoring Position Note. Indicative anchoring positions are for reference only and should not be used as waypoints. 34° 32.1’S 136° 16.3’E The best position for anchoring depends on many factors including vessel draft, tide, and forecast wind. Homestead Bay (or Reevesby Lagoon as it is often called) is on the western side of the largest island in the group. It is a favourite anchorage for many sailors, and usually the first stop for visitors to the group. -

Current Status of Western Blue Groper at Selected Locations in South Australia: Results of the 2014 Survey and Comparison with Historical Surveys
Current status of western blue groper at selected locations in South Australia: results of the 2014 survey and comparison with historical surveys Report to the Conservation Council of South Australia James Brook 2014 Brook (2014) Status of western blue groper in South Australia This publication should be cited as: Brook, J. (2014). Current status of western blue groper at selected locations in South Australia: results of the 2014 survey and comparison with historical surveys. Report to the Conservation Council of South Australia. J Diversity Pty Ltd, Adelaide. Cover photo: Male western blue groper (Achoerodus gouldii) at the Investigator Group, 2006. Photo credit: James Brook. Disclaimer The findings and opinions expressed in this publication are those of the author and do not necessarily reflect those of the Conservation Council of South Australia. While reasonable efforts have been made to ensure the contents of this report are factually correct, the Conservation Council of South Australia and the author do not accept responsibility for the accuracy and completeness of the contents. The author does not accept liability for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this report. 2 Brook (2014) Status of western blue groper in South Australia Acknowledgements The current report was funded by an Australian Government Community Environment Grant through the Conservation Council of South Australia (CCSA). Thanks are due to: • Alex Gaut (CCSA), Steve Leske (CCSA/Reef Watch), and all of the volunteers who collected the data • Dr Simon Bryars, who was a sounding board for discussion and reviewed a draft of the report • Dr Scoresby Shepherd AO (SARDI) for providing historical data and reviewing a draft of the report. -

Enrolment and Election Booklet 2020-21
The Aboriginal Land Council of Tasmania Enrolment and election booklet 2020-21 Includes: Procedures and guidelines June 2020 Contents THE ABORIGINAL LAND COUNCIL OF TASMANIA (ALCT) ............................... 3 Definitions of the 5 electoral areas THE ALCT ELECTORS ROLL ........................................................................ 4 The Preliminary Roll Who is entitled to be on the Roll Objections to transfer from the Preliminary Roll to the Roll GUIDELINES CONCERNING THE REQUIREMENTS SET OUT IN SECTION 3A OF THE ABORIGINAL LANDS ACT 1995 ..................................................................... 6 Aboriginal ancestry Self-identification Communal recognition PROCEDURE FOR DEALING WITH OBJECTIONS TO ENROLMENT ....................... 8 ELECTORAL COMMISSIONER’S REVIEW COMMITTEE ...................................... 9 CONDUCT OF ALCT ELECTIONS ................................................................. 10 How to stand as a candidate for election Eligibility to stand How to vote How votes are counted Declaration of the result Term of office Filling a casual vacancy 2020 - 21 ALCT ELECTION TIMETABLE ...................................... BACK COVER 2020-21 ALCT Election Procedures & Guidelines June 2020 2 The Aboriginal Land Council of Tasmania The Aboriginal Lands Act 1995 (the Act) provides for the election of members of the Aboriginal Land Council of Tasmania (ALCT). ALCT holds and manages Aboriginal land on behalf of the Aboriginal people of Tasmania. The ALCT comprises 8 members, elected for a term of approximately -

Special Issue3.7 MB
Volume Eleven Conservation Science 2016 Western Australia Review and synthesis of knowledge of insular ecology, with emphasis on the islands of Western Australia IAN ABBOTT and ALLAN WILLS i TABLE OF CONTENTS Page ABSTRACT 1 INTRODUCTION 2 METHODS 17 Data sources 17 Personal knowledge 17 Assumptions 17 Nomenclatural conventions 17 PRELIMINARY 18 Concepts and definitions 18 Island nomenclature 18 Scope 20 INSULAR FEATURES AND THE ISLAND SYNDROME 20 Physical description 20 Biological description 23 Reduced species richness 23 Occurrence of endemic species or subspecies 23 Occurrence of unique ecosystems 27 Species characteristic of WA islands 27 Hyperabundance 30 Habitat changes 31 Behavioural changes 32 Morphological changes 33 Changes in niches 35 Genetic changes 35 CONCEPTUAL FRAMEWORK 36 Degree of exposure to wave action and salt spray 36 Normal exposure 36 Extreme exposure and tidal surge 40 Substrate 41 Topographic variation 42 Maximum elevation 43 Climate 44 Number and extent of vegetation and other types of habitat present 45 Degree of isolation from the nearest source area 49 History: Time since separation (or formation) 52 Planar area 54 Presence of breeding seals, seabirds, and turtles 59 Presence of Indigenous people 60 Activities of Europeans 63 Sampling completeness and comparability 81 Ecological interactions 83 Coups de foudres 94 LINKAGES BETWEEN THE 15 FACTORS 94 ii THE TRANSITION FROM MAINLAND TO ISLAND: KNOWNS; KNOWN UNKNOWNS; AND UNKNOWN UNKNOWNS 96 SPECIES TURNOVER 99 Landbird species 100 Seabird species 108 Waterbird -

Stansbury Basin
136°30'E 137°0'E 137°30'E 138°0'E 138°30'E 139°0'E 700000 PEL 126 800000 900000 6300000 6300000 Caroona Creek (CP) 33°30'S Munyaroo (CP) The Plug Range (CP) Caroona Creek (CP) PEL 126 Clements Gap (CP) PEL 606 Mokota (CP) 33°30'S Yeldulknie (CP) Middlecamp Hills (CP) Cleve " PL1 " Cowell Burra " Red Banks (CP) Franklin Harbor (CP) 573 Clare " Wallaroo " Lochiel Spring Gully (CP) Kadina " Hopkins Creek (CP) " Bird Islands (CP) 34°0'S Bird Islands (CP)Moonta " PEL 120 34°0'S " Clinton (CP) Spencer Gulf "Wakefield 266 PEL 606 Wills Creek (CP) 6200000 Maitland Kapunda 6200000 " " 574 Brookfield (CP) Goose Island (CP) PEL 174 Nuriootpa 34°30'S " Penrice " PL1 34°30'S 266 Gawler " 266 Swan Reach (CP) Sir Joseph Banks Group (CP) Kaiserstuhl (CP) PL6 Franklin Harbor (CP) Port Gawler (CP) Elizabeth Minlaton 266 " " Ramsay (CP) Torrens Island (CP) Mount Pleasant " Torrens Island (CP) Cromer (CP) Leven Beach (CP) Minlacowie (CP) ADELAIDE Mannum " " Carribie (CP) Charleston (CP) 35°0'S Yorketown " Kenneth Stirling (CP) Ettrick (CP) Gambier Islands (CP) Edithburgh 35°0'S " Gulf of St Vincent Mark Oliphant (CP) Warrenben (CP) Scott Creek (CP) Murray Bridge Long Island (RP) Troubridge Island (CP) " Point Davenport (CP) Onkaparinga River (NP) Innes (NP) Moana Sands (CP) Monarto (CP) 6100000 6100000 Strathalbyn Kyeema (CP) " Ferries - McDonald (CP) PL13 Aldinga Scrub (CP) " Cox Scrub (CP) Althorpe Islands (CP) Poonthie Ruwi - Riverdale (CP) Yulte (CP) Milang Tolderol (GR) Scott (CP) " Myponga (CP) 35°30'S Currency Creek (GR) " Granite Island (RP) 35°30'S -

(Haliaeetus Leucogaster) and the Eastern Osprey (Pandion Cristatus
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 37 - PART 1 - March - 2011 Journal of The South Australian Ornithological Association Inc. In this issue: Osprey and White-bellied Sea-Eagle populations in South Australia Birds of Para Wirra Recreation Park Bird report 2009 March 2011 1 Distribution and status of White-bellied Sea-Eagle, Haliaeetus leucogaster, and Eastern Osprey, Pandion cristatus, populations in South Australia T. E. DENNIS, S. A. DETmAR, A. V. BROOkS AND H. m. DENNIS. Abstract Surveys throughout coastal regions and in the INTRODUCTION Riverland of South Australia over three breeding seasons between May 2008 and October 2010, Top-order predators, such as the White-bellied estimated the population of White-bellied Sea- Sea-Eagle, Haliaeetus leucogaster, and Eastern Eagle, Haliaeetus leucogaster, as 70 to 80 pairs Osprey, Pandion cristatus, are recognised and Eastern Osprey, Pandion cristatus, as 55 to indicator species by which to measure 65 pairs. Compared to former surveys these data wilderness quality and environmental integrity suggest a 21.7% decline in the White-bellied Sea- in a rapidly changing world (Newton 1979). In Eagle population and an 18.3% decline for Eastern South Australia (SA) both species have small Osprey over former mainland habitats. Most (79.2%) populations with evidence of recent declines sea-eagle territories were based on offshore islands linked to increasing human activity in coastal including Kangaroo Island, while most (60.3%) areas (Dennis 2004; Dennis et al. 2011 in press). osprey territories were on the mainland and near- A survey of the sea-eagle population in the shore islets or reefs. The majority of territories were mid 1990s found evidence for a decline in the in the west of the State and on Kangaroo Island, with breeding range since European colonisation three sub-regions identified as retaining significant (Dennis and Lashmar 1996). -

080058-89.02.017.Pdf
t9l .Ig6I pup spu?Fr rr"rl?r1mv qnos raq1oaqt dq panqs tou pus 916I uao^\teq sluauennboJ puu surelqord lusue8suuur 1eneds wq sauo8a uc .fu1mpw snorru,r aql uI luar&(oldua ',uq .(tg6l a;oJareqt puuls1oore8ueltr 0t dpo 1u reted lS sr ur saiuzqc aql s,roqs osIB elqeJ srqt usrmoJ 'urpilsny 'V'S) puels tseSrul geu oq; ur 1sa?re1p4ql aql 3o luetugedeq Z alq?J rrr rtr\oqs su padoldua puu prr"lsr aroqsJJorrprJpnsnv qlnos lsJErel aql ruJ ere,u eldoed ZS9 I feqf pa roqs snsseC srllsll?ls dq r ?olp sp qlr^\ puplsl oorp8ue1 Jo neomg u"rl?Jlsnv eql uo{ sorn8g luereJ lsour ,u1 0g€ t Jo eW T86I ul puu 00S € ,{lel?urxorddu sr uoqelndod '(derd ur '8ur,no:8 7r luosaJd eqJ petec pue uoqcnpord ,a uosurqoU) uoqecrJqndro3 peredard Smeq ,{11uermc '(tg6l )potsa^q roJ perualo Suraq puel$ oql Jo qJnu eru sda,r-rnsaseql3o EFSer pa[elop aqJ &usJ qlvrr pedolaaap fuouoce Surqsg puu Surure; e puu pue uosurqo;) pegoder useq aleq pesn spoporu palles-er su,rr prrqsr eql sreo,{ Eurpeet:ns eq1 re,ro prru s{nser druuruqord aqt pue (puep1 ooreSuqtr rnq 698I uI peuopwqs sE^\ elrs lrrrod seaeell eql SumnJcxa) sprrelsl aroqsJJo u"{e4snv qlnos '998I raqueJeo IIl eprelepv Jo tuaruslDesIeuroJ oqt aql Jo lsou uo palelduor ueeq A\ou aaeq s,{e,rrns aro3aq ,(ueduo3 rr"4u4snv qtnos qtgf 'oAE eqt dq ,{nt p:6o1org sree,{009 6 ol 000 L uea r1aq palulosr ur slors8rry1 u,no1paserd 6rll J?eu salaed 'o?e ;o lrrrod ererrirspuulsr Surura sr 3ql Jo dllJolpu aq; sree,{ paqslqplse peuuurad lE s?a\lueurep1os uuedorng y 009 0I spuplsl dpearg pue uosr"ed pup o8e srea,{ 'seruolocuorJ-"3s rel?l puB -
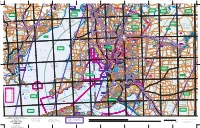
S P E N C E R G U L F S T G U L F V I N C E N T Adelaide
Yatala Harbour Paratoo Hill Turkey 1640 Sunset Hill Pekina Hill Mt Grainger Nackara Hill 1296 Katunga Booleroo "Avonlea" 2297 Depot Hill Creek 2133 Wilcherry Hill 975 Roopena 1844 Grampus Hill Anabama East Hut 1001 Dawson 1182 660 Mt Remarkable SOUTH Mount 2169 440 660 (salt) Mt Robert Grainger Scobie Hill "Mazar" vermin 3160 2264 "Manunda" Wirrigenda Hill Weednanna Hill Mt Whyalla Melrose Black Rock Goldfield 827 "Buckleboo" 893 729 Mambray Creek 2133 "Wyoming" salt (2658±) RANGE Pekina Wheal Bassett Mine 1001 765 Station Hill Creek Manunda 1073 proof 1477 Cooyerdoo Hill Maurice Hill 2566 Morowie Hill Nackara (abandoned) "Bulyninnie" "Oak Park" "Kimberley" "Wilcherry" LAKE "Budgeree" fence GILLES Booleroo Oratan Rock 417 Yeltanna Hill Centre Oodla "Hill Grange" Plain 1431 "Gilles Downs" Wirra Hillgrange 1073 B pipeline "Wattle Grove" O Tcharkuldu Hill T Fullerville "Tiverton 942 E HWY Outstation" N Backy Pt "Old Manunda" 276 E pumping station L substation Tregalana Baroota Yatina L Fitzgerald Bay A Middleback Murray Town 2097 water Ucolta "Pitcairn" E Buckleboo 1306 G 315 water AN Wild Dog Hill salt Tarcowie R Iron Peak "Terrananya" Cunyarie Moseley Nobs "Middleback" 1900 works (1900±) 1234 "Lilydale" H False Bay substation Yaninee I Stoney Hill O L PETERBOROUGH "Blue Hills" LC L HWY Point Lowly PEKINA A 378 S Iron Prince Mine Black Pt Lancelot RANGE (2294±) 1228 PU 499 Corrobinnie Hill 965 Iron Baron "Oakvale" Wudinna Hill 689 Cortlinye "Kimboo" Iron Baron Waite Hill "Loch Lilly" 857 "Pualco" pipeline Mt Nadjuri 499 Pinbong 1244 Iron -

Overview of Tasmania's Offshore Islands and Their Role in Nature
Papers and Proceedings of the Royal Society of Tasmania, Volume 154, 2020 83 OVERVIEW OF TASMANIA’S OFFSHORE ISLANDS AND THEIR ROLE IN NATURE CONSERVATION by Sally L. Bryant and Stephen Harris (with one text-figure, two tables, eight plates and two appendices) Bryant, S.L. & Harris, S. 2020 (9:xii): Overview of Tasmania’s offshore islands and their role in nature conservation.Papers and Proceedings of the Royal Society of Tasmania 154: 83–106. https://doi.org/10.26749/rstpp.154.83 ISSN: 0080–4703. Tasmanian Land Conservancy, PO Box 2112, Lower Sandy Bay, Tasmania 7005, Australia (SLB*); Department of Archaeology and Natural History, College of Asia and the Pacific, Australian National University, Canberra, ACT 2601 (SH). *Author for correspondence: Email: [email protected] Since the 1970s, knowledge of Tasmania’s offshore islands has expanded greatly due to an increase in systematic and regional surveys, the continuation of several long-term monitoring programs and the improved delivery of pest management and translocation programs. However, many islands remain data-poor especially for invertebrate fauna, and non-vascular flora, and information sources are dispersed across numerous platforms. While more than 90% of Tasmania’s offshore islands are statutory reserves, many are impacted by a range of disturbances, particularly invasive species with no decision-making framework in place to prioritise their management. This paper synthesises the significant contribution offshore islands make to Tasmania’s land-based natural assets and identifies gaps and deficiencies hampering their protection. A continuing focus on detailed gap-filling surveys aided by partnership restoration programs and collaborative national forums must be strengthened if we are to capitalise on the conservation benefits islands provide in the face of rapidly changing environmental conditions and pressure for future use. -

South Australian Ornithologist
- THE: South Australian Ornithologist. VOL. XIV.] AFIUL 1, 1938. [Part 6. Notes on some Birds seen on Flinders and other islands off the Eyre Peninsula coasts, February March, 1937. By H. H. Finlayson. The birds noted below wereseen during 11 stay of some three weeks in February of the year 1937 on Flinders. Island and during shorter visits to some other islands of neighbouring coasts and waters. The observations are of a random and desultory kind, being made incidentally to enquiry into the mammal fauna of the places named, and do not relate to more than a small part of the total avifauna, nor include even all the prominent forms. With the exception of Prof. J. B. Cleland's paper on the birds of Pearson's Island (Trans. Roy. Soc. S.A., Vol. XLVII, 119/ 126, 1923), little seems to have been published on the birds of the smaller islands of the South Australian coasts, and even such fragmentary records as the following, provided they are made at first hand, may have value as contributions to the completed lists of the future. Flinders Island has an. area of about 9,000 acres, and lies about 18 miles west of Elliston on the Eyre Peninsula mainland. It has the structure, usual hereabouts, of a limestone platform resting on granite base, which is exposed at the waterline. The coastline is varied and is occupied by cliffs, boulder-shingle, and sand beach in about equal proportion. The soil is in general a shallow light sandy .loam, although firmer red soils occur on the south-west side. -

Eyre Peninsula OCEAN 7
Mount F G To Coober Pedy (See Flinders H I Olympic Dam J Christie Ranges and Outback Region) Village Andamooka Wynbring Roxby Downs To Maralinga Lyons Tarcoola WOOMERA PROHIBITED AREA (Restricted Entry) Malbooma 87 Lake Lake Younghusband Ferguson Patricia 1 1 Glendambo Kingoonya Lake Hanson Lake Torrens 'Coondambo' Lake Yellabinna Regional Kultanaby Lake Hart National Park Lake Harris Wirramn na Woomera Reserve Lake Torre Lake Lake Gairdner Pimba Windabout ns Everard National Park Pernatty Lagoon Island Wirrappa See map on opposite Lake page for continuation Lagoon Dog Fence 'Oakden Hill' Gairdner 'Mahanewo' 2 Yumbarra Conservation Stuart 2 Park 'Lake Everard' Lake Finnis 'Kangaroo To Nundroo (See OTC Earth Wells' 'Yalymboo' Lake Dutton map opposite) Station Bookaloo Pureba Conservation 'Moonaree' Lake Highway Penong 57 1 'Kondoolka' Lake MacFarlane Park 16 Acraman 'Yadlamalka' 59 Nunnyah Con. Res Ceduna 'Yudnapinna' Mudlamuckla 'Hiltaba' Jumpuppy Hesso To Hawker (See Bay Hill Flinders and Koolgera Cooria Hill Outback Region) Point Bell 41 Con. Res 87 Murat Smoky Mt. Gairdner 51 Goat Is. Bay 93 33 Unalla Hill 'Low Hill' St. Peter Smoky Bay Horseshoe Evans Is. Gawler Tent Hill Quorn Island 29 27 Barkers Hill 'Cariewerloo' Hill Eyre Wirrulla 67 'Yardea' 'Mount Ive' Nuyts Archipelago Is. Conservation Haslam 'Thurlga' 50 'Nonning' Park Flinders Port Augusta St. Francis Point Brown 76 Paney Hill Red Hill Streaky 46 25 3 Island 75 61 3 Bay Eyre 'Paney' Ranges Highway Army 27 Harris Bluff 'Corunna' Training Cape Bauer 39 28 43 Poochera Lake Area Olive Island 61 Iron Knob 33 45 Gilles Corvisart Highway 'Buckleboo' Streaky Bay Lake Gilles 47 Bay Minnipa Pinkawillinie Conservation Park 1 56 17 31 Point Westall 21 Highway Conservation Buckleboo 21 35 43 Eyre Sceale Bay 17 Park Iron Baron 88 Cape Blanche 43 13 27 23 Wudinna 24 24 Port Germein Searcy Bay Kulliparu Kyancutta 18 Whyalla Point Labatt Port Kenny 22 Con. -

Sir Joseph Banks Islands Discovery Voyage
Sir Joseph Banks Islands Discovery Voyage Port Lincoln - Port Adelaide 28 January – 1 February 2018 Contact Details: Marketing & Bookings 0432 495 603 [email protected] Friends of the One & All Sailing Ship Inc One and All PO Box 3214 is a registered not for profit charity Port Adelaide SA 5015 Each ticket booked supports the www.oneandallship.com.au Youth Development Programs Tall Ship Sailing Adventure Be on deck… Come on board and see South Australia's greatest sailing asset. Walk the decks, be at the ship's helm, venture into the galley & the saloon. This handcrafted ship will tell you the stories of yesteryear of how sail transport & life on board was like. The STV One & All is a living and working vessel providing unique experiences for all. Here is the opportunity to join this 5 Day voyage to be part of the ship’s 30th year of service to the community! This unique voyage will set sail to the remote and beautiful Sir Joseph Banks Group of Islands. We will sail across the Gulf while the “locals” come out to play. The seals, dolphins and sea birds are never far away to watch and follow our moves. The wildlife take every opportunity to give us a show of their nimble moves above or below the waterline. On this great adventure, the gems of these remote islands will show a history captured in time of the early settlements and native scrub lands. Our South Australian coastline will keep unfolding to features such as Althorpe Island, Wedge Island, the foot of Eyre Peninsular and the many lighthouses that dot our coast.