The Respiratory System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Prospective Isolation of NKX2-1–Expressing Human Lung Progenitors Derived from Pluripotent Stem Cells
The Journal of Clinical Investigation RESEARCH ARTICLE Prospective isolation of NKX2-1–expressing human lung progenitors derived from pluripotent stem cells Finn Hawkins,1,2 Philipp Kramer,3 Anjali Jacob,1,2 Ian Driver,4 Dylan C. Thomas,1 Katherine B. McCauley,1,2 Nicholas Skvir,1 Ana M. Crane,3 Anita A. Kurmann,1,5 Anthony N. Hollenberg,5 Sinead Nguyen,1 Brandon G. Wong,6 Ahmad S. Khalil,6,7 Sarah X.L. Huang,3,8 Susan Guttentag,9 Jason R. Rock,4 John M. Shannon,10 Brian R. Davis,3 and Darrell N. Kotton1,2 2 1Center for Regenerative Medicine, and The Pulmonary Center and Department of Medicine, Boston University School of Medicine, Boston, Massachusetts, USA. 3Center for Stem Cell and Regenerative Medicine, Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center, Houston, Texas, USA. 4Department of Anatomy, UCSF, San Francisco, California, USA. 5Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA. 6Department of Biomedical Engineering and Biological Design Center, Boston University, Boston, Massachusetts, USA. 7Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts, USA. 8Columbia Center for Translational Immunology & Columbia Center for Human Development, Columbia University Medical Center, New York, New York, USA. 9Department of Pediatrics, Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee, USA. 10Division of Pulmonary Biology, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA. It has been postulated that during human fetal development, all cells of the lung epithelium derive from embryonic, endodermal, NK2 homeobox 1–expressing (NKX2-1+) precursor cells. -

The Diseases of Airway-Tracheal Diverticulum: a Review of the Literature
Review Article The diseases of airway-tracheal diverticulum: a review of the literature Asli Tanrivermis Sayit, Muzaffer Elmali, Dilek Saglam, Cetin Celenk Department of Radiology, Faculty of Medicine, Ondokuz Mayis University, Samsun, Turkey Contributions: (I) Conception and design: A Tanrivermis Sayit; (II) Administrative support: M Elmali, C Celenk; (III) Provision of study materials or patients: A Tanrivermis Sayit; (IV) Collection and assembly of data: A Tanrivermis Sayit, D Saglam; (V) Data analysis and interpretation: A Tanrivermis Sayit, M Elmali, C Celenk; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Asli Tanrivermis Sayit. Department of Radiology, Faculty of Medicine, Ondokuz Mayis University, 55139, Atakum/Samsun, Turkey. Email: [email protected]. Abstract: Tracheal diverticulum (DV) is a type of paratracheal air cyst (PTAC) that is often asymptomatic and usually detected incidentally by imaging methods. Tracheal DV are divided into two subgroups: congenital and acquired. Dysphagia, odynophagia, neck pain, hoarseness, hemoptysis, choking, and recurrent episodes of hiccups and burping can also be seen in symptomatic patients. Thin-section multidetector computed tomography (MDCT) is useful for diagnosis of tracheal diverticulum. The relationship between DV and tracheal lumen can be demonstrated by axial, coronal, and sagittal reformat multiplanar images. Bronchoscopy can also be used in diagnosis for tracheal DV. However, the connection between DV and tracheal lumen can not be shown easily with bronchoscopy. Conservative treatment is the preferred treatment in asymptomatic patients. Surgical or conservative treatment can be performed for symptomatic patients, depending on patient age and physical condition. Keywords: Trachea; diverticulum (DV); thorax; multidetector computed tomography; tracheal diseases; chronic obstructive pulmonary disease (CODP) Submitted Sep 17, 2016. -

PQI 15 Asthma in Younger Adults Admission Rate
AHRQ Quality Indicators™ (AHRQ QI™) ICD-10-CM/PCS Specification v2021 Prevention Quality Indicator 15 (PQI 15) Asthma in Younger Adults Admission Rate July 2021 Area-Level Indicator Type of Score: Rate Prepared by: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services www.qualityindicators.ahrq.gov DESCRIPTION Admissions for a principal diagnosis of asthma per 100,000 population, ages 18 to 39 years. Excludes admissions with an indication of cystic fibrosis or anomalies of the respiratory system, obstetric admissions, and transfers from other institutions. [NOTE: The software provides the rate per population. However, common practice reports the measure as per 100,000 population. The user must multiply the rate obtained from the software by 100,000 to report admissions per 100,000 population.] NUMERATOR Discharges, for patients ages 18 through 39 years, with a principal ICD-10-CM diagnosis code for asthma (ACSASTD*). [NOTE: Obstetric discharges are not included in the PQI rate for PQI 15, though the AHRQ QI™ does not explicitly exclude obstetric cases. By definition, discharges with a principal diagnosis of asthma exclude obstetric discharges, because the principal diagnosis for an obstetric discharge would identify it as obstetric, and no such diagnoses are included in the set of qualifying diagnoses.] July 2021 1 of 5 AHRQ QI™ ICD-10-CM/PCS Specification v2021 PQI 15 Asthma in Younger Adults Admission Rate www.qualityindicators.ahrq.gov NUMERATOR EXCLUSIONS Exclude cases: • with any listed ICD-10-CM -

Embryology, Comparative Anatomy, and Congenital Malformations of the Gastrointestinal Tract
Edorium J Anat Embryo 2016;3:39–50. Danowitz et al. 39 www.edoriumjournals.com/ej/ae REVIEW ARTICLE PEER REVIEWED | OPEN ACCESS Embryology, comparative anatomy, and congenital malformations of the gastrointestinal tract Melinda Danowitz, Nikos Solounias ABSTRACT Human digestive development is an essential topic for medical students and physicians, Evolutionary biology gives context to human and many common congenital abnormalities embryonic digestive organs, and demonstrates directly relate to gastrointestinal embryology. how structural adaptations can fit changing We believe this comprehensive review of environmental requirements. Comparative gastrointestinal embryology and comparative anatomy is rarely included in the medical anatomy will facilitate a better understanding of school curriculum. However, its concepts gut development, congenital abnormalities, and facilitate a deeper comprehension of anatomy adaptations to various evolutionary ecological and development by putting the morphology conditions. into an evolutionary perspective. Features of gastrointestinal development reflect the transition Keywords: Anatomy education, Digestive, Embry- from aquatic to terrestrial environments, such as ology, Gastrointestinal tract the elongation of the colon in land vertebrates, allowing for better water reabsorption. In How to cite this article addition, fishes exhibit ciliary transport in the esophagus, which facilitates particle transport in Danowitz M, Solounias N. Embryology, comparative water, whereas land mammals develop striated anatomy, and congenital malformations of the and smooth esophageal musculature and utilize gastrointestinal tract. Edorium J Anat Embryo peristaltic muscle contractions, allowing for 2016;3:39–50. better voluntary control of swallowing. The development of an extensive vitelline drainage system to the liver, which ultimately creates Article ID: 100014A04MD2016 the adult hepatic portal system allows for the evolution of complex hepatic metabolic ********* functions seen in many vertebrates today. -

Regulation of Early Lung Morphogenesis: Questions, Facts and Controversies
REVIEW 1611 Development 133, 1611-1624 (2006) doi:10.1242/dev.02310 Regulation of early lung morphogenesis: questions, facts and controversies Wellington V. Cardoso* and Jining Lü During early respiratory system development, the foregut endodermal specification, lung primordium formation, and the endoderm gives rise to the tracheal and lung cell progenitors. regulation of the initial stages of branching morphogenesis and Through branching morphogenesis, and in coordination with differentiation in the embryonic lung. We address questions such as vascular development, a tree-like structure of epithelial ‘when and how is respiratory cell fate established?’, ‘how do lung tubules forms and differentiates to produce the airways and buds form?’, ‘how are stereotypical patterns of airway branching and alveoli. Recent studies have implicated the fibroblast growth cellular diversity generated in the developing lung?’ and ‘which factor, sonic hedgehog, bone morphogenetic protein, retinoic pathways and targets are key to these processes?’. Most of what is acid and Wnt signaling pathways, and various transcription described refers to mouse lung development because of the genetic factors in regulating the initial stages of lung development. data available (Table 1). Lung vascular development and later events, However, the precise roles of these molecules and how they such as sacculation and alveoli formation, are not discussed in this interact in the developing lung is subject to debate. Here, we review (for reviews, see Pauling and Vu, 2004; Williams, -

Branching Morphogenesis of the Lung: New Molecular Insights Into an Old Problem
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang1 and Andrew P. McMahon2 1Cardiovascular Research Institute, University of California, San Francisco, CA 94143, USA 2Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA It has been known for decades that branching morpho- This process coincides with the appearance of another genesis of the lung is mediated through reciprocal inter- endodermal derivative, the dorsal pancreatic bud pri- actions between the epithelium and its underlying mordium, whereas the liver and thyroid bud emerge one mesenchyme. In recent years, several key players, in day earlier from the ventral foregut endoderm [5]. The particular members of the major signaling pathways lung primordium is composed of two parts: the future that mediate this interaction, have been identified. Here, trachea and two endodermal buds (primary buds), which we review the genetic and molecular studies of these give rise to the left and right lobes of the distal lung. Both key components, which have provided a conceptual components are composed of an epithelial layer of endo- framework for understanding the interactions of these derm surrounded by splanchnic lateral plate mesoderm major signaling pathways in branching morphogenesis. cells. Initially the primary buds grow ventrally and The future challenge is to translate understanding of caudally, and initiate lateral branches at invariant posi- the signaling cascade into knowledge of the cellular tions, beginning around 10.5 dpc. In this way, five responses, including cell proliferation, migration and secondary buds are generated, four on the right side and differentiation, that lead to the stereotyped branching.* one on the left side, leading to the formation of four right lobes and one left lobe of the mature lung in mice. -

Embryology Dr. Azal N.Al-Nusear Respiratory System 1-Upper
Embryology Dr. Azal N.Al-Nusear Respiratory System 1-Upper Respiratory System: The upper respiratory system consists of the nose, nasopharynx, and oropharynx. 2-Lower Respiratory system: The lower respiratory system consists of the larynx, trachea, bronchi, and lungs. The first sign of development is the formation of the respiratory diverticulum in the ventral wall of the primitive foregut during week 4. The distal end of the respiratory diverticulum enlarges to form the lung bud. The lung bud divides into two bronchial buds that branch into the primary, secondary, tertiary, and subsegmental bronchi. The respiratory diverticulum initially is in open communication with the foregut, but eventually they become separated by mesoderm (tracheoesophageal folds). When the tracheoesophageal folds fuse in the midline to form the tracheoesophageal septum, the foregut is divided into the trachea ventrally and esophagus dorsally. RD: respiratory diverticulum F: foregut. VM: visceral mesoderm. TEF : tracheoesophageal folds the trachea (T) and esophagus (E). B = bronchial buds. LL = left lung; L = right lung; Development of Individual Parts of the Respiratory System Larynx The larynx develops from the cranial part of laryngotracheal diverticulum. The opening of the respiratory diverticulum into the foregut becomes the laryngeal orifice. The mesenchyme (of fourth and sixth pharyngeal arches) surrounding the laryngeal orifice proliferates. As a result, the slit-like laryngeal orifice becomes T shaped. Subsequently laryngeal orifice acquires a characteristic adult shape. The lining epithelium of larynx develops from endoderm of this diverticulum. At first the endodermal cells proliferate and completely obliterate lumen of larynx. Later the cells breakdown and recanalization of larynx take place. -
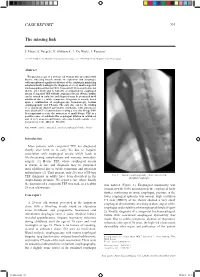
The Missing Link CASE REPORT
CASE REPORT 531 The missing link J. Maus1, S. Naegels1, H. Slabbynck2, L. De Waele1, I. Ruytjens1 (1) ZNA Middelheim, Department of Gastro-enterology ; (2) ZNA Middelheim, Department of Pneumology. Abstract We present a case of a 28-year old woman who presented with bizarre wheezing breath sounds on expiration and dysphagia, with unexplained significant dilation of the esophagus mimicking achalasia finally leading to the diagnosis of a very small congenital tracheoesophageal fistula (TEF). Congenital TEF is usually detected shortly after birth and is typically accompanied by esophageal atresia. Congenital TEF without esophageal atresia (H-type fistula) can be missed in early life and diagnosis may be postponed until adulthood due to subtle symptoms. Diagnosis is usually based upon a combination of esophagoscopy, bronchoscopy, barium esophagography and CT-scan. The only clue can be the finding of a significant dilated aperistaltic esophagus, with subsequent more detailed CT reconstruction revealing a very tiny H-type TEF. It is important to raise the awareness of small H-type TEF as a possible cause of achalasia-like esophageal dilation in adulthood and of very unusual and bizarre wheezing breath sounds. (Acta gastroenterol. belg., 2018, 81, 531-533). Key words : adult ; congenital ; tracheoesophageal fistula ; H-type. Introduction Most patients with congenital TEF are diagnosed shortly after birth or in early life due to frequent association with esophageal atresia which leads to life-threatening complications and warrants immediate surgery. (1) H-type TEF, where esophageal atresia is absent, is rare and diagnosis may be postponed until adulthood due to subtle symptoms and physician unfamiliarity (2). -

Gastroesophageal Reflux: Anatomy and Physiology
26th Annual Scientific Conference | May 1-4, 2017 | Hollywood, FL Gastroesophageal Reflux: Anatomy and Physiology Amy Lowery Carroll, MSN, RN, CPNP- AC, CPEN Children’s of Mississippi at The University of Mississippi Medical Center Jackson, Mississippi Disclosure Information I have no disclosures. Objectives • Review embryologic development of GI system • Review normal anatomy and physiology of esophagus and stomach • Review pathophysiology of Gastroesophageal Reflux 1 26th Annual Scientific Conference | May 1-4, 2017 | Hollywood, FL Embryology of the Gastrointestinal System GI and Respiratory systems are derived from the endoderm after cephalocaudal and lateral folding of the yolk sack of the embryo Primitive gut can be divided into 3 sections: Foregut Extends from oropharynx to the liver outgrowth Thyroid, esophagus, respiratory epithelium, stomach liver, biliary tree, pancreas, and proximal portion of duodenum Midgut Liver outgrowth to the transverse colon Develops into the small intestine and proximal colon Hindgut Extends from transverse colon to the cloacal membrane and forms the remainder of the colon and rectum Forms the urogenital tract Embryology of the Gastrointestinal System Respiratory epithelium appears as a bud of the esophagus around 4th week of gestation Tracheoesophageal septum develops to separate the foregut into ventral tracheal epithelium and dorsal esophageal epithelium Esophagus starts out short and lengthens to final extent by 7 weeks Anatomy and Physiology of GI System Upper GI Tract Mouth Pharynx Esophagus Stomach -

Dnmt1 Is Required for Proximal-Distal Patterning of the Lung Endoderm and for Restraining Alveolar Type 2 Cell Fate
Developmental Biology 454 (2019) 108–117 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Original research article Dnmt1 is required for proximal-distal patterning of the lung endoderm and for restraining alveolar type 2 cell fate Derek C. Liberti a,b,c,1, Jarod A. Zepp b,c,1, Christina A. Bartoni b,c, Kyle H. Liberti d, Su Zhou c, Minmin Lu c, Michael P. Morley b,c, Edward E. Morrisey a,b,c,e,f,* a Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA b Penn Cardiovascular Institute, University of Pennsylvania, Philadelphia, PA, 19104, USA c Penn Center for Pulmonary Biology, University of Pennsylvania, Philadelphia, PA, 19104, USA d Middleware Engineering, Red Hat, Westford, MA, 01886, USA e Department of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA f Penn-CHOP Lung Biology Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, 19104, USA ABSTRACT Lung endoderm development occurs through a series of finely coordinated transcriptional processes that are regulated by epigenetic mechanisms. However, the role of DNA methylation in regulating lung endoderm development remains poorly understood. We demonstrate that DNA methyltransferase 1 (Dnmt1) is required for early branching morphogenesis of the lungs and for restraining epithelial fate specification. Loss of Dnmt1 leads to an early branching defect, a loss of epithelial polarity and proximal endodermal cell differentiation, and an expansion of the distal endoderm compartment. Dnmt1 deficiency also disrupts epithelial-mesenchymal crosstalk and leads to precocious distal endodermal cell differentiation with premature expression of alveolar type 2 cell restricted genes. -
Development of Respiratory System
Development of respiratory system Respiratory block-Anatomy-Lecture 5 Editing file Color guide : Only in boys slides in Green Only in girls slides in Purple Objectives important in Red Doctor note in Blue Extra information in Grey ➔ Identify the development of the laryngotracheal (respiratory) diverticulum. ➔ Identify the development of the larynx. ➔ Identify the development of the trachea. ➔ Identify the development of the bronchi & Lungs. ➔ Describe the periods of the maturation of the lung. ➔ Identify the most congenital anomaly 3 Respiratory system Upper respiratory Lower respiratory tract tract Nasal cavity & Nose paranasal Laryngopharynx Larynx Trachea Bronchi Lungs sinuses Development of the respiratory tract 4 The endoderm & Begins during the 4th week of Development of longitudinal Proximal & distal parts of the surrounding splanchnic development tracheoesophageal septum respiratory diverticulum mesoderm ▹ Divides the diverticulum ▹ Begins as a median ▹ The proximal part of the ▹ The endoderm lining the into: outgrowth (laryngotracheal respiratory diverticulum laryngotracheal ▸ Dorsal portion*: groove) from the caudal part remains tubular and forms diverticulum (respiratory primordium (in the of the ventral wall of the larynx & trachea. diverticulum) gives rise to earliest stage of primitive pharynx (foregut). ▹ The distal end of the the: development) of the ▹ The groove invaginates (fold diverticulum dilates to form ▸ Epithelium & glands oropharynx & within itself) and forms lung bud, which divides to of the respiratory esophagus. laryngotracheal give rise to 2 lung buds tract. ▸ Ventral portion*: (respiratory) diverticulum. (primary bronchial buds). ▹ The surrounding splanchnic primordium (=give mesoderm gives rise to the: rise) of larynx, ▸ Connective tissue, trachea, bronchi & cartilage & smooth lungs. muscles of the respiratory tract. * Remember that the larynx, trachea, bronchi & lungs lie anteriorly while the oropharynx & esophagus lie posteriorly.