Balancing the Costs of Carbon Gain and Water Transport: Testing a New Theoretical Framework for Plant Functional Ecology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Excursion Report July 2019
July 2019 Number 193 In this issue... Excursion report July 2019..................................................1 Cooktown Botanic Gardens Work Party 2019...............................1 Species List .........................................5 Australian Native Plants Society (Australia) - 2019 Conference............................................8 Excursion report Blooming Biodiversity............8 Albany, Western Australia July 2019 - 29th September to 4 October 2019..............................8 Cooktown Botanic Gardens Work Party 2019 Mystery Styphelia Update.......8 Don Lawie and Stuart Worboys What's Happening.........................9 Cooktown Botanic Gardens is Queensland's Cairns Branch.............................9 northern-most botanic gardens. Established Townsville Branch....................9 not long after the town's birth in 1873, the gardens are home to historic stone-pitched Tablelands Branch...................9 waterways. and grand and unusual heritage trees. They also lie a short distance from the Endeavour River, where in 1770 Cook beached his damaged ship for repairs after an unfortunate interaction with the Great Barrier Reef. Page 1 During their sevenSGAP Cairnsweek enforcedBranch - Newsletterstay, the 193 botanist Joseph Banks, naturalist wet season plus much work has Daniels Solander and their party resulted in a green outlook of surveyed and collected more neat graveled pathways, low key extensively than anywhere else on but effective fencing, and trees, their voyage, making Cooktown shrubs and vines all thriving and arguably the birthplace of well mulched. An efficient but Australian scientific botany. unobtrusive “pop-up” watering Since 1987, Cooktown Botanic system has assisted with Gardens has been the semi-regular maintaining growth. mid-year destination for Cairns and Tablelands branches of SGAP. Over the decades, we have assisted with tasks across the gardens, from weeding to planting, from mulching to bridge renovations. -

Flora.Sa.Gov.Au/Jabg
JOURNAL of the ADELAIDE BOTANIC GARDENS AN OPEN ACCESS JOURNAL FOR AUSTRALIAN SYSTEMATIC BOTANY flora.sa.gov.au/jabg Published by the STATE HERBARIUM OF SOUTH AUSTRALIA on behalf of the BOARD OF THE BOTANIC GARDENS AND STATE HERBARIUM © Board of the Botanic Gardens and State Herbarium, Adelaide, South Australia © Department of Environment, Water and Natural Resources, Government of South Australia All rights reserved State Herbarium of South Australia PO Box 2732 Kent Town SA 5071 Australia © 2012 Board of the Botanic Gardens & State Herbarium, Government of South Australia J. Adelaide Bot. Gard. 25 (2012) 71–96 © 2012 Department of Environment, Water and Natural Resources, Govt of South Australia Notes on Hibbertia (Dilleniaceae) 8. Seven new species, a new combination and four new subspecies from subgen. Hemistemma, mainly from the central coast of New South Wales H.R. Toelkena & R.T. Millerb a State Herbarium of South Australia, DENR Science Resource Centre, P.O. Box 2732, Kent Town, South Australia 5071 E-mail: [email protected] b 13 Park Road, Bulli, New South Wales 2516 E-mail: [email protected] Abstract Increased collections from the Hibbertia-rich vicinity of Sydney, New South Wales, prompted a survey of rarer species to publicise the need for more information ahead of the rapid urban spread. Many of these species were previously misunderstood or are listed as rare and endangered. Thirteen new taxa (in bold) are described and discussed in context with the following seventeen taxa within seven different species groups: 1. H. acicularis group: H. woronorana Toelken; 2. H. humifusa group: H. -

Native Plant Species List
Understorey Network Clarence Plant Species List This plant species list is a sample of species that occur in your municipality and are relatively easy to grow or to purchase from a native plant nursery. Some of the more common plants are listed, as well as uncommon species that have a limited distribution and only occur in your area. However, many more species could be included on the list. Observing your local bush is a good way to get an idea of what else may be grown in your area and is suited to your property. To help choose your plants, each species is scored against soil type, vegetation community and uses. An extensive listing of suitable species can be found on the NRM South and Understorey Network websites. (white correa) Correa alba Clarence Coastal Vegetation Coastal Rainforest Eucalypt Forest Wet Woodland and Dry Eucalypt Forest Vegetation Grassy Heath Wetland Sedgeland and Riparian Vegetation Montane drained soil Well drained soil Poorly Sandy soil Loamy soil Clay soil soil Poor soil Fertile Low flammablity Erosion control Shelter belts Bush tucker Wise Water Salinity control Easy to propagate from seed Easy to propagate from cuttings Easy to propagate by division Standard Common Grow Vegetation Community Soil Type Uses from Name Name Endemic Trees Acacia mearnsii black wattle • • • • • • • • • • • Acacia verticillata prickly mimosa • • • • • • • • • • • • • Allocasuarina littoralis black sheoak • • • • • • • • • Allocasuarina verticillata drooping sheoak • • • • • • • • • • Banksia marginata silver banksia • • • • • • • -

Brooklyn, Cloudland, Melsonby (Gaarraay)
BUSH BLITZ SPECIES DISCOVERY PROGRAM Brooklyn, Cloudland, Melsonby (Gaarraay) Nature Refuges Eubenangee Swamp, Hann Tableland, Melsonby (Gaarraay) National Parks Upper Bridge Creek Queensland 29 April–27 May · 26–27 July 2010 Australian Biological Resources Study What is Contents Bush Blitz? Bush Blitz is a four-year, What is Bush Blitz? 2 multi-million dollar Abbreviations 2 partnership between the Summary 3 Australian Government, Introduction 4 BHP Billiton and Earthwatch Reserves Overview 6 Australia to document plants Methods 11 and animals in selected properties across Australia’s Results 14 National Reserve System. Discussion 17 Appendix A: Species Lists 31 Fauna 32 This innovative partnership Vertebrates 32 harnesses the expertise of many Invertebrates 50 of Australia’s top scientists from Flora 62 museums, herbaria, universities, Appendix B: Threatened Species 107 and other institutions and Fauna 108 organisations across the country. Flora 111 Appendix C: Exotic and Pest Species 113 Fauna 114 Flora 115 Glossary 119 Abbreviations ANHAT Australian Natural Heritage Assessment Tool EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) NCA Nature Conservation Act 1992 (Queensland) NRS National Reserve System 2 Bush Blitz survey report Summary A Bush Blitz survey was conducted in the Cape Exotic vertebrate pests were not a focus York Peninsula, Einasleigh Uplands and Wet of this Bush Blitz, however the Cane Toad Tropics bioregions of Queensland during April, (Rhinella marina) was recorded in both Cloudland May and July 2010. Results include 1,186 species Nature Refuge and Hann Tableland National added to those known across the reserves. Of Park. Only one exotic invertebrate species was these, 36 are putative species new to science, recorded, the Spiked Awlsnail (Allopeas clavulinus) including 24 species of true bug, 9 species of in Cloudland Nature Refuge. -
Abelmoschus Moschatus Subsp
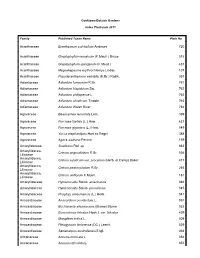
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Native Plants Sixth Edition Sixth Edition AUSTRALIAN Native Plants Cultivation, Use in Landscaping and Propagation
AUSTRALIAN NATIVE PLANTS SIXTH EDITION SIXTH EDITION AUSTRALIAN NATIVE PLANTS Cultivation, Use in Landscaping and Propagation John W. Wrigley Murray Fagg Sixth Edition published in Australia in 2013 by ACKNOWLEDGEMENTS Reed New Holland an imprint of New Holland Publishers (Australia) Pty Ltd Sydney • Auckland • London • Cape Town Many people have helped us since 1977 when we began writing the first edition of Garfield House 86–88 Edgware Road London W2 2EA United Kingdom Australian Native Plants. Some of these folk have regrettably passed on, others have moved 1/66 Gibbes Street Chatswood NSW 2067 Australia to different areas. We endeavour here to acknowledge their assistance, without which the 218 Lake Road Northcote Auckland New Zealand Wembley Square First Floor Solan Road Gardens Cape Town 8001 South Africa various editions of this book would not have been as useful to so many gardeners and lovers of Australian plants. www.newhollandpublishers.com To the following people, our sincere thanks: Steve Adams, Ralph Bailey, Natalie Barnett, www.newholland.com.au Tony Bean, Lloyd Bird, John Birks, Mr and Mrs Blacklock, Don Blaxell, Jim Bourner, John Copyright © 2013 in text: John Wrigley Briggs, Colin Broadfoot, Dot Brown, the late George Brown, Ray Brown, Leslie Conway, Copyright © 2013 in map: Ian Faulkner Copyright © 2013 in photographs and illustrations: Murray Fagg Russell and Sharon Costin, Kirsten Cowley, Lyn Craven (Petraeomyrtus punicea photograph) Copyright © 2013 New Holland Publishers (Australia) Pty Ltd Richard Cummings, Bert -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

Comparisons of Vegetation Recovery Post Fire, Logging and Salvage Logging in the Victorian Central Highlands
Comparisons of vegetation recovery post fire, logging and salvage logging in the Victorian Central Highlands David Paul Blair November 2018 A thesis submitted for the degree of Doctor of Philosophy at The Australian National University © Copyright by David Blair 2018 All Rights Reserved Candidate’s declaration This thesis contains no material that has been accepted for the award of any other degree or diploma in any university. The research, analysis and writing in the thesis are substantially (>90%) my own work. To the best of my knowledge, it contains no material previously published or written by another person, except where due reference is made in the text. 10 November, 2018 David Blair Date ACKNOWLEDGEMENTS I wish to acknowledge the Traditional Owners of the Central Highlands, the land on which my research was conducted. I pay my respects to their Elders, past and present. I wish to thank the many people who supported me and gave me the opportunity to achieve this degree. It is only through standing on their strong, caring and supportive shoulders that I have had the privilege to gain a higher and more focused view of our amazing world. To my supervisors, Prof. David Lindenmayer, Dr. Sam Banks and Dr. Annabel Smith, thank you for guiding me along my path of discovery and allowing me space to explore. Your encouragement, belief in me, and genuine friendships have made the long tough road enjoyable. Special thanks also to Lachie McBurney. It’s been fantastic working with you over the years and knowing should I need some shade cloth, a rope, winch or quality night vision goggles, you will always be there for me. -

EIS MEWF Vol 3 App16
Mount Emerald Wind Farm, Herberton Range North Queensland Environmental Impact Statement Volume 3 Appendix 16 Mount Emerald Wind Farm Flora Report (R72444) Prepared by RPS PR100246 / R72894; Volume 3 – Final November 2014 Mount Emerald Wind Farm Flora Report Prepared by: Prepared for: RPS AUSTRALIA EAST PTY LTD RATCH AUSTRALIA CORPORATION LIMITED 135 Abbott Street PO Box 1949 Level 4 CAIRNS QLD 4870 231 George Street BRISBANE QLD 4000 T: +61 4031 1336 F: +61 4031 29142 T: +61 7 3214 36400 E: [email protected] F: +61 2 8913 9423 E: Client Manager: Mellissa Jess W: www.ratchaustralia.com Report Number: PR100246-1 / R72444 Version / Date: Draft September 2013 rpsgroup.com.au Mount Emerald Wind Farm Flora Report IMPORTANT NOTE Apart from fair dealing for the purposes of private study, research, criticism, or review as permitted under the Copyright Act, no part of this report, its attachments or appendices may be reproduced by any process without the written consent of RPS Australia East Pty Ltd. All enquiries should be directed to RPS Australia East Pty Ltd. We have prepared this report for the sole purposes of RATCH Australia Corporation Limited (“Client”) for the specific purpose of only for which it is supplied (“Purpose”). This report is strictly limited to the purpose and the facts and matters stated in it and does not apply directly or indirectly and will not be used for any other application, purpose, use or matter. In preparing this report we have made certain assumptions. We have assumed that all information and documents provided to us by the Client or as a result of a specific request or enquiry were complete, accurate and up-to-date. -

In This Issue: Contents
In this issue: Save the Date… Contents • Next meeting: Monthly Meeting Recap - June ........................ 2 • Wednesday, August 23, 7.30pm August Meeting Teaser: ................................... 5 Plant Profile: ..................................................... 6 Eucalyptus formanii ...................................... 6 Gibberellic acid (GA) ......................................... 8 Upcoming Events ............................................ 10 Contacts:......................................................... 12 Chris Lindorff spoke to us last year on Woodland Birds, including setting up an Upcoming Meetings: area for attracting them. This month, Chris will return and expand on setting up a - September 27 – sanctuary to attract wildlife Bonsai - October 8 – Nature Coming Up... walk thru Brisbane Ranges • Lots of other events, refer to pages 10 and 11 for more… - October 25 – AGM & Flower Table - November 22 – End Of Year & Photo Comp 1 | P a g e Monthly Meeting Recap - June Our first day meeting was held with a result of only 11 members able to attend. I can say with certainty that those who were absent missed out on a fabulous talk by Kathryn FitzGibbon, who is employed by the Melton City Council as a landscape architect. There were plenty of Awww’s as Kathryn showed us her humble beginnings in the garden as she recalled many hours helping her dad as she was growing up. Kathryn continued her love of gardening, formalising it by obtaining a Bachelor of Design and Master of Landscape Architecture at RMIT, although she admitted that it was a course about the ‘feelings’ of plants, and not actually about plants themselves. Her hard work paid off as she was part of a team that won a Gold Medal at the Grand Designs Live Exhibition in 2012 for their structure incorporating succulents. -

Honey and Pollen Flora of SE Australia Species
List of families - genus/species Page Acanthaceae ........................................................................................................................................................................34 Avicennia marina grey mangrove 34 Aizoaceae ............................................................................................................................................................................... 35 Mesembryanthemum crystallinum ice plant 35 Alliaceae ................................................................................................................................................................................... 36 Allium cepa onions 36 Amaranthaceae ..................................................................................................................................................................37 Ptilotus species foxtails 37 Anacardiaceae ................................................................................................................................................................... 38 Schinus molle var areira pepper tree 38 Schinus terebinthifolius Brazilian pepper tree 39 Apiaceae .................................................................................................................................................................................. 40 Daucus carota carrot 40 Foeniculum vulgare fennel 41 Araliaceae ................................................................................................................................................................................42