Notes on the Diet of a Jerdon's Leafbird Chloropsis Jerdoni Chick
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sri Lanka: January 2015
Tropical Birding Trip Report Sri Lanka: January 2015 A Tropical Birding CUSTOM tour SRI LANKA: Ceylon Sojourn 9th- 23rd January 2015 Tour Leaders: Sam Woods & Chaminda Dilruk SRI LANKA JUNGLEFOWL is Sri Lanka’s colorful national bird, which was ranked among the top five birds of the tour by the group. All photos in this report were taken by Sam Woods. 1 www.tropicalbirding.com +1-409-515-0514 [email protected] Page Tropical Birding Trip Report Sri Lanka: January 2015 INTRODUCTION In many ways Sri Lanka covers it all; for the serious birder, even those with experience from elsewhere in the Indian subcontinent, it offers up a healthy batch of at least 32 endemic bird species (this list continues to grow, though, so could increase further yet); for those without any previous experience of the subcontinent it offers these but, being an island of limited diversity, not the overwhelming numbers of birds, which can be intimidating for the first timer; and for those with a natural history slant that extends beyond the avian, there is plentiful other wildlife besides, to keep all happy, such as endemic monkeys, strange reptiles only found on this teardrop-shaped island, and a bounty of butterflies, which feature day-in, day-out. It should also be made clear that while it appears like a chunk of India which has dropped of the main subcontinent, to frame it, as merely an extension of India, would be a grave injustice, as Sri Lanka feels, looks, and even tastes very different. There are some cultural quirks that make India itself, sometimes challenging to visit for the westerner. -

Egg Recognition in Cinereous Tits (Parus Cinereus): Eggshell Spots Matter Jianping Liu1 , Canchao Yang1 , Jiangping Yu2,3 , Haitao Wang2,4 and Wei Liang1*
Liu et al. Avian Res (2019) 10:37 https://doi.org/10.1186/s40657-019-0178-1 Avian Research RESEARCH Open Access Egg recognition in Cinereous Tits (Parus cinereus): eggshell spots matter Jianping Liu1 , Canchao Yang1 , Jiangping Yu2,3 , Haitao Wang2,4 and Wei Liang1* Abstract Background: Brood parasitic birds such as cuckoos (Cuculus spp.) can reduce their host’s reproductive success. Such selection pressure on the hosts has driven the evolution of defense behaviors such as egg rejection against cuckoo parasitism. Studies have shown that Cinereous Tits (Parus cinereus) in China have a good ability for recognizing foreign eggs. However, it is unclear whether egg spots play a role in egg recognition. The aims of our study were to inves- tigate the egg recognition ability of two Cinereous Tit populations in China and to explore the role of spots in egg recognition. Methods: To test the efect of eggshell spots on egg recognition, pure white eggs of the White-rumped Munia (Lon- chura striata) and eggs of White-rumped Munia painted with red brown spots were used to simulate experimental parasitism. Results: Egg experiments showed that Cinereous Tits rejected 51.5% of pure white eggs of the White-rumped Munia, but only 14.3% of spotted eggs of the White-rumped Munia. There was a signifcant diference in egg recognition and rejection rate between the two egg types. Conclusions: We conclude that eggshell spots on Cinereous Tit eggs had a signaling function and may be essential to tits for recognizing and rejecting parasitic eggs. Keywords: Brood parasitism, Egg recognition, Egg rejection, Eggshell spots, Parus cinereus Background egg rejection by hosts, many parasitic birds evolve coun- Te mutual adaptations and counter-defense strategies ter-adaptations to overcome the hosts’ defenses by laying between brood parasitic birds such as cuckoos (Cuculus mimicking (Brooke and Davies 1988; Avilés et al. -

India: Kaziranga National Park Extension
INDIA: KAZIRANGA NATIONAL PARK EXTENSION FEBRUARY 22–27, 2019 The true star of this extension was the Indian One-horned Rhinoceros (Photo M. Valkenburg) LEADER: MACHIEL VALKENBURG LIST COMPILED BY: MACHIEL VALKENBURG VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM INDIA: KAZIRANGA NATIONAL PARK EXTENSION February 22–27, 2019 By Machiel Valkenburg This wonderful Kaziranga extension was part of our amazing Maharajas’ Express train trip, starting in Mumbai and finishing in Delhi. We flew from Delhi to Guwahati, located in the far northeast of India. A long drive later through the hectic traffic of this enjoyable country, we arrived at our lodge in the evening. (Photo by tour participant Robert Warren) We enjoyed three full days of the wildlife and avifauna spectacles of the famous Kaziranga National Park. This park is one of the last easily accessible places to find the endangered Indian One-horned Rhinoceros together with a healthy population of Asian Elephant and Asiatic Wild Buffalo. We saw plenty individuals of all species; the rhino especially made an impression on all of us. It is such an impressive piece of evolution, a serious armored “tank”! On two mornings we loved the elephant rides provided by the park; on the back of these attractive animals we came very close to the rhinos. The fertile flood plains of the park consist of alluvial silts, exposed sandbars, and riverine flood-formed lakes called Beels. This open habitat is not only good for mammals but definitely a true gem for some great birds. Interesting but common birds included Bar-headed Goose, Red Junglefowl, Woolly-necked Stork, and Lesser Adjutant, while the endangered Greater Adjutant and Black-necked Stork were good hits in the stork section. -

Assam Extension I 17Th to 21St March 2015 (5 Days)
Trip Report Assam Extension I 17th to 21st March 2015 (5 days) Greater Adjutant by Glen Valentine Tour leaders: Glen Valentine & Wayne Jones Trip report compiled by Glen Valentine Trip Report - RBT Assam Extension I 2015 2 Top 5 Birds for the Assam Extension as voted by tour participants: 1. Pied Falconet 4. Ibisbill 2. Greater Adjutant 5. Wedge-tailed Green Pigeon 3. White-winged Duck Honourable mentions: Slender-billed Vulture, Swamp Francolin & Slender-billed Babbler Tour Summary: Our adventure through the north-east Indian subcontinent began in the bustling city of Guwahati, the capital of Assam province in north-east India. We kicked off our birding with a short but extremely productive visit to the sprawling dump at the edge of town. Along the way we stopped for eye-catching, introductory species such as Coppersmith Barbet, Purple Sunbird and Striated Grassbird that showed well in the scopes, before arriving at the dump where large frolicking flocks of the endangered and range-restricted Greater Adjutant greeted us, along with hordes of Black Kites and Eastern Cattle Egrets. Eastern Jungle Crows were also in attendance as were White Indian One-horned Rhinoceros and Citrine Wagtails, Pied and Jungle Mynas and Brown Shrike. A Yellow Bittern that eventually showed very well in a small pond adjacent to the dump was a delightful bonus, while a short stroll deeper into the refuse yielded the last remaining target species in the form of good numbers of Lesser Adjutant. After our intimate experience with the sought- after adjutant storks it was time to continue our journey to the grassy plains, wetlands, forests and woodlands of the fabulous Kaziranga National Park, our destination for the next two nights. -

Comparative Analysis of Hissing Calls in Five Tit Species Li Zhang, Jianping Liu, Zezhong Gao, Lei Zhang, Dongmei Wan, Wei Liang, Anders Pape Møller
Comparative analysis of hissing calls in five tit species Li Zhang, Jianping Liu, Zezhong Gao, Lei Zhang, Dongmei Wan, Wei Liang, Anders Pape Møller To cite this version: Li Zhang, Jianping Liu, Zezhong Gao, Lei Zhang, Dongmei Wan, et al.. Comparative analysis of hissing calls in five tit species. Behavioural Processes, Elsevier, 2019. hal-03024634 HAL Id: hal-03024634 https://hal-cnrs.archives-ouvertes.fr/hal-03024634 Submitted on 25 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Comparative analysis of hissing calls in five tit species 1 2 1 1 1, * 2 Li Zhang , Jianping Liu , Zezhong Gao , Lei Zhang , Dongmei Wan , Wei 2, * 3 3 Liang , Anders Pape Møller 1 4 Key Laboratory of Animal Resource and Epidemic Disease Prevention, 5 College of Life Sciences, Liaoning University, Shenyang 110036, China 2 6 Ministry of Education Key Laboratory for Ecology of Tropical Islands, 7 College of Life Sciences, Hainan Normal University, Haikou 571158, China 3 8 Ecologie Systématique Evolution, Université Paris-Sud, CNRS, 9 AgroParisTech, Université Paris-Saclay, F-91405 Orsay Cedex, France 10 Email address: 11 LZ, [email protected], ID: 0000-0003-1224-7855 12 JL, [email protected], ID: 0000-0001-6526-8831 13 ZG, [email protected] 14 LZ, [email protected], ID: 0000-0003-2328-1194 15 DW, [email protected], ID: 0000-0002-1465-6110 16 WL, [email protected], ID: 0000-0002-0004-9707 17 APM, [email protected], ID: 0000-0003-3739-4675 18 Word count: 4571 19 *Corresponding author. -

DIVERSITY of BIRDS ACROSS LAND USE and HABITAT GRADIENTS in FORESTS, RUBBER AGROFORESTS and RUBBER PLANTATIONS of NORTH SUMATRA Asep Ayat1,* and Hesti L
Indonesian Journal of Forestry Research Vol. 2, No. 2, October 2015, 103-120 ISSN: 2355-7079 / E-ISSN: 2406-8195 DIVERSITY OF BIRDS ACROSS LAND USE AND HABITAT GRADIENTS IN FORESTS, RUBBER AGROFORESTS AND RUBBER PLANTATIONS OF NORTH SUMATRA Asep Ayat1,* and Hesti L. Tata2 1Burung Indonesia, Jalan Dadali 32, Bogor 16161, Indonesia 2Forest Research and Development Center, Jl. Gunung Batu 5, Bogor, Indonesia Received: 31 March 2014, Revised: 10 May 2014, Accepted: 11 October 2015 DIVERSITY OF BIRDS ACROSS LAND USE AND HABITAT GRADIENTS IN FORESTS, RUBBER AGROFORESTS AND RUBBER PLANTATIONS OF NORTH SUMATRA. Birds play a pivotal role in the ecosystem, but in disturbed areas their roles may be limited due to the changes of their natural habitats. This paper studies the birds' habitats in Simalungun and Asahan Districts, North Sumatra. The study was conducted in four habitats: natural forest, rubber agroforests, rubber monoculture plantations and emplacement areas. The birds were observed using descriptive survey methods by implementing a quick biodiversity survey, data were collected along one km transect. The results showed that in total, 142 species of birds from 42 families were observed in the four habitats. Natural forests had the highest diversity of bird species, followed by rubber agroforests, emplacement areas and rubber plantations, with a Shannon-Wiener index of 3.8, 3.6, 3.0 and 2.9, respectively. Regarding the IUCN red list species, 12 bird species of near- threatened status and 2 species of vulnerable status were recorded. Based on CITES categories, one species was listed in the Appendix I, 12 species were classified in Appendix II and 26 bird species were protected under Indonesian regulations. -

Inference of Phylogenetic Relationships in Passerine Birds (Aves: Passeriformes) Using New Molecular Markers
Institut für Biochemie und Biologie Evolutionsbiologie/Spezielle Zoologie Inference of phylogenetic relationships in passerine birds (Aves: Passeriformes) using new molecular markers Dissertation zur Erlangung des akademischen Grades “doctor rerum naturalium” (Dr. rer. nat.) in der Wissenschaftsdisziplin “Evolutionsbiologie“ eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät der Universität Potsdam von Simone Treplin Potsdam, August 2006 Acknowledgements Acknowledgements First of all, I would like to thank Prof. Dr. Ralph Tiedemann for the exciting topic of my thesis. I’m grateful for his ongoing interest, discussions, support, and confidence in the project and me. I thank the University of Potsdam for the opportunity to perform my PhD and the financial and logistical funds. This thesis would not have been possible without many institutions and people, who provided samples: University of Kiel, Haustierkunde (Heiner Luttmann and Joachim Oesert), Zoologischer Garten Berlin (Rudolf Reinhard), Tierpark Berlin (Martin Kaiser), Transvaal Museum, South Africa (Tamar Cassidy), Vogelpark Walsrode (Bernd Marcordes), Eberhard Curio, Roger Fotso, Tomek Janiszewski, Hazell Shokellu Thompson, and Dieter Wallschläger. Additionally, I thank everybody who thought of me in the moment of finding a bird, collected and delivered it immediately. I express my gratitude to Christoph Bleidorn for his great help with the phylogenetic analyses, the fight with the cluster, the discussions, and proof-reading. Special thanks go to Susanne Hauswaldt for patiently reading my thesis and improving my English. I thank my colleagues of the whole group of evolutionary biology/systematic zoology for the friendly and positive working atmosphere, the funny lunch brakes, and the favours in the lab. I’m grateful to Romy for being my first, ‘easy-care’ diploma-student and producing many data. -

Answer Key Primary (Rainforest in a City Book)
Answer Key Primary (Rainforest in a City Book) Fun Activities Name : _________________________( ) Class: _________ Date: ______ Primary Worksheet 1 Rainforest in a City: Word Search Fun Here are some words extracted from the book “Rainforest in the City”. See if you can find them in the word search below. Have fun! CANOPY TROPICAL FOREST RAIN ENVIRONMENT JELUTONG OWL HOT BUTTERFLY MONKEY SNAKE TREES MERANTI CRICKETS ANT FLORA NOCTURNAL MILLIPEDES AMAZING LAYERS N Q D R B N Q U U W O J B R G T J I O M L A B C Z D N O G E P N A Y H Z C B G I N C A U L U K L T R X J N H T S Z N R A M D N H G U G O T Z D H U E B M U F A H N B N T N W R T C I R E L I C S Z W W J Z O K L E O M D N D H L A A I C I J F N Q Z E T J S A A H L X T N M O Q O G Z B S Z L W L J S I G Z G B R F R U L X Z J P E D K B P T G J H U J E V U E X U V V L G X E A J M O T D S M P P P V V C C V Q D Y L F T U X T L U G Y F J R Z V J E A H G M A O G M E R A N T I O I W S Z N M O N K E Y N P T B X C F L O R A L A Y E R S Y W E R W M K C A N O P Y E N V I R O N M E N T E A N T V K S T R O P I C A L I R L T B U T T E R F L Y S N A K E T C J S Primary Worksheet 2 Rainforest in a City: Word Scramble Fun Here are some words extracted from the book “Rainforest in the City”. -

Sri Lanka Ceylon Sojourn
Sri Lanka Ceylon Sojourn A Tropical Birding Set Departure January 20 – February 2, 2019 Guides: Ken Behrens & Saman Kumara Report and photos by Ken Behrens TOUR SUMMARY The Indian Subcontinent is rich, both in human culture and history and in biological treasures. Sri Lanka is a large island at the southern tip of this region, lying a short distance from the Indian mainland. It contains a rich selection of the birds, mammals, and other wildlife of the subcontinent, which thrive in a selection of delightful protected areas; enough to thoroughly recommend it as a destination for a travelling birder. But even more alluringly, Sri Lanka is home to dozens of endemic birds – 33 given current Clements taxonomy, though this number is sure to continue to climb as distinctive subspecies are split as full species. Sri Lanka has decent infrastructure, excellent food, good lodges, and wonderfully kind and hospitable people. This short and sweet tour is equally attractive to those eager for their first taste of the Indian subcontinent, or to those who have travelled it extensively, and want to see the island’s endemic birds. As on all of our tours in recent years, we “cleaned up” on the endemics, enjoying great views of all 33 of them. This set of endemics includes a bunch of delightful birds, such as Sri Lanka Junglefowl, Sri Lanka Spurfowl, Serendib Scops-Owl, Chestnut-backed Owlet, Sri Lanka Hanging-Parrot, Red-faced Malkoha, Crimson-backed Woodpecker, Green-billed Coucal, Sri Sri Lanka: Ceylon Sojourn January 20-February 2, 2019 Lanka Blue Magpie, Sri Lanka (Scaly) and Spot-winged Thrushes, Yellow-eared Bulbul, and White-throated (Legge’s) Flowerpecker. -

No 491 Winter 2018
No 491 Winter 2018 Photograph - Jay in December - Penn, Wolverhampton Photographer Ellis Partridge This front page is sponsored by The Birder’s Store, Worcester WMBC News Is published in March, June, September and December each year to link members with each other, what’s been happening, current issues and forthcoming events on the birding scene in our area and further afield together with a selection of your articles and a comprehensive summary of the recorded bird sightings in our area This issue of your Newsletter has, for me, been an “embarrassment of riches”, so much news, so many issues your Management Committee want to inform you about and so many fascinating articles on your birding exploits, thank you all so much. When compiling I have to limit myself to 44 pages as to include more would put us over the 100g limit for second class post (which when you are sending out 1700 copies is quite significant). Faced with this dilemma decisions had to be made about what to leave out. My decision was to include all the text, so you will find less photographs than usual this time, and my apologies therefore go to those whose shots I have had to leave out this time. There are two main reasons for the increase in copy. The first, of course, is the number of excellent articles being written by our members - please keep these coming, I am quite happy to face this problem again next issue and I am constantly being told by members I meet how much they enjoy your articles. -
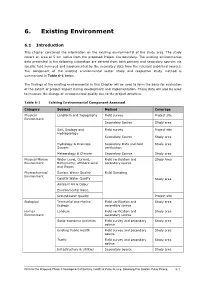
6. Existing Environment
___________________________________________________________________________________________ 6. Existing Environment 6.1 Introduction This chapter contained the information on the existing environmental of the study area. The study covers an area of 5 km radius from the proposed Project site boundary. The existing environmental data presented in the following subsection are derived from both primary and secondary sources via specific field surveyed and supplemented by the secondary data from the relevant published sources. The component of the existing environmental sector study and respective study method is summarised in Table 6-1 below. The findings of the existing environmental in this Chapter will be used to form the basis for evaluation of the extent of project impact during development and implementation. These data will also be used to measure the change of environmental quality due to the project activities. Table 6-1 Existing Environmental Component Assessed Category Subject Method Coverage Physical Landform and Topography Field survey Project site Environment Secondary Source Study area Soil, Geology and Field survey Project site Hydrogeology Secondary Source Study area Hydrology & Drainage Secondary Data and field Study area System verification Meteorology & Climate Secondary Source Study area Physical Marine Water Level, Current, Field verification and Study Area Environment Bathymetry, offshore wind secondary source and Waves Physiochemical Surface Water Quality Field Sampling Environment Coastal Water Quality Study area -

Kanha Bird Checklist (Pdf)
KANHA BIRD SURVEY - CHECKLIST Expected Species eBird 'English (India)' Local name eBird scientific name status Detection 17/03 18/03 19/03 Lesser Whistling Duck Dendrocygna javanica R Ruddy Shelduck Tadorna ferruginea W Cotton Pygmy-Goose Nettapus coromandelianus R Gadwall Anas strepera W Eurasian Wigeon Anas penelope W Indian Spot-billed Duck Anas poecilorhyncha R Northern Pintail Anas acuta W Garganey Anas querquedula W Green-winged Teal (Common Teal) Anas crecca W Indian Peafowl Pavo cristatus R Red Spurfowl Galloperdix spadicea R Jungle Bush-Quail Perdicula asiatica R Painted Francolin Francolinus pictus R H Grey Francolin Francolinus pondicerianus R H Red Junglefowl Gallus gallus R H Little Grebe Tachybaptus ruficollis R Asian Openbill Anastomus oscitans R Black Stork Ciconia nigra W Woolly-necked Stork Ciconia episcopus R Lesser Adjutant Leptoptilos javanicus R Little Cormorant Microcarbo niger R Great Cormorant Phalacrocorax carbo R Indian Cormorant (Indian Shag) Phalacrocorax fuscicollis R Oriental Darter Anhinga melanogaster R Grey Heron Ardea cinerea R Purple Heron Ardea purpurea R Great Egret Ardea alba R Intermediate Egret Mesophoyx intermedia R Little Egret Egretta garzetta R Cattle Egret Bubulcus ibis R Indian Pond-Heron Ardeola grayii R Threskiornis Black-headed Ibis melanocephalus R Red-naped Ibis (Indian Black Ibis) Pseudibis papillosa R Black-shouldered Kite Elanus caeruleus R (Black-winged Kite) Crested (Oriental) Honey Buzzard Pernis ptilorhynchus R White-rumped Vulture Gyps bengalensis R Indian Vulture (Indian Long-billed