The Efficacy of Jianpi Yiqi Therapy for Chronic Atrophic Gastritis: a Systematic Review and Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Network Pharmacology and Traditional Chinese Medicine: Devel- Opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1*
ISSN: 2377-3634 Lu et al. Int J Diabetes Clin Res 2017, 4:077 DOI: 10.23937/2377-3634/1410077 Volume 4 | Issue 2 International Journal of Open Access Diabetes and Clinical Research REVIEW ARTICLE Network Pharmacology and Traditional Chinese Medicine: Devel- opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1* 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China Check for 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China updates *Corresponding author: Yitao Chen, MD, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected]; Changyu Li, MD, College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected] Abstract Research and Development Dilemma for Anti- Diabetes Drugs Partly due to the failure of single-target drugs, diabetes mel- litus, a chronic metabolic disease with complex pathogene- Type 2 Diabetes Mellitus (T2DM), generally agreed sis and long-term medication requirements, is increasing in to be caused by insulin resistance and/or insulin defi- prevalence worldwide and urgently needs multi-component and multi-target treatments. Traditional Chinese herbs are ciency, constitutes almost 95 percent of all diabetes the principal drug of Chinese medicine, which is effective cases [4]. Because of the pathogenesis, the mainstre- against diabetes. However, Chinese herbs’ mechanism of am anti-diabetic drugs are insulin secretagogues (sul- action is difficult to elucidate due to its multiple components phonylureas and meglitinide analogues), insulin sensi- and multi-target effects. -

Supplementary Materials 1
Supplementary materials 1 Table S1 The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Botanical Pin Yin Name Form Ingredients Recommendation for daily intake (g) preparations (汉语) Plant food supplements (PFS) Si Ji Kang Mei Yang Xin Yuan -Rou Dou Kou xylooligosaccharide, isomalt, nutmeg (myristica PFS 1 Fu He Tang Pian tablet 4 tablets (1.4 g) fragrans), galangal, cinnamon, chicken gizzards (四季康美养心源-肉豆蔻复合糖片) Ai Si Meng Hui Xiang fennel seed, figs, prunes, dates, apples, St.Johns 2-4 tablets (2.8-5.6 g) PFS 2 Fu He Pian tablet Breed, jamaican ginger root (爱司盟茴香复合片) Zi Ran Mei Xiao Hui Xiaong Jiao Nang foeniculi powder, cinnamomi cortex, papaya PFS 3 capsule concentrated powder, green oat concentrated powder, 3 capsules (1.8 g) (自然美小茴香胶囊) brewer’s yeast, cabbage, monkey head mushroom An Mei Qi Hui Xiang Cao Ben Fu He Pian fennel seed, perilla seed, cassia seed, herbaceous PFS 4 tablet 1-2 tablets (1.4-2.8 g) (安美奇茴香草本复合片) complex papaya enzymes, bromelain enzymes, lactobacillus An Mei Qi Jiao Su Xian Wei Ying Yang Pian acidophilus, apple fiber, lemon plup fiber, fennel PFS 5 tablet seed, cascara sagrada, jamaican ginger root, herbal 2 tablets (2.7 g) (安美奇酵素纤维营养片) support complex (figs, prunes, dates, apples, St. Johns bread) Table S1 (continued) The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Pin Yin Name Botanical Form Ingredients Recommendation for daily intake (g) preparations (汉语) Gan Cao Pian glycyrrhiza uralensis, licorice -

Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 1-18-2012 Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats Zhiying Dou Tianjin University of Traditional Chinese Medicine Kefeng Li Michigan Technological University Ping Wang Tianjin University of Traditional Chinese Medicine Liu Cao Tianjin University of Traditional Chinese Medicine Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Recommended Citation Dou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules, 17(1), 951-970. http://doi.org/10.3390/molecules17010951 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1969 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Molecules 2012, 17, 951-970; doi:10.3390/molecules17010951 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats Zhiying Dou 1,*, Kefeng Li 2, Ping Wang 1 and Liu Cao 1 1 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China 2 Department of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-22-5959-6235. Received: 29 November 2011; in revised form: 5 January 2012 / Accepted: 9 January 2012 / Published: 18 January 2012 Abstract: Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. -

NRDC: Generally Recognized As Secret
NRDC Report April 2014 Generally Recognized as Secret: Chemicals Added to Food in the United States Tom Neltner, J.D., Maricel Maffini, Ph.D. Natural Resources Defense Council In April 2014, the Natural Resources Defense Council (NRDC) released a report raising concerns about a loophole in the Food Additives Amendment of 1958 for substances designated by food manufacturers as “generally recognized as safe” (GRAS). The report identified 56 companies that appeared to market 275 chemicals for use in food based on undisclosed GRAS safety determinations. For each chemical we identified in this study, we did not find evidence that FDA had cleared them for use in food. The 1958 law exempted from the formal, extended FDA approval process common food ingredients like vinegar and vegetable oil whose use qualifies as GRAS. It may have appeared reasonable at the time, but that exemption has been stretched into a loophole that has swallowed the law. The exemption allows manufacturers to make safety determinations that the uses of their newest chemicals in food are safe without notifying the FDA. The agency’s attempts to limit these undisclosed GRAS determinations by asking industry to voluntarily inform the FDA about their chemicals are insufficient to ensure the safety of our food in today’s global marketplace with a complex food supply. Furthermore, no other developed country in the world has a system like GRAS to provide oversight of food ingredients. In Table 1 and 2 of the report, NRDC identified the 56 companies and the number of chemicals that each company appeared to market as GRAS without FDA clearance. -
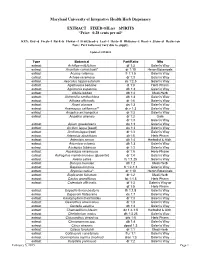
SPIRITS *Price: 0.28 Cents Per Ml*
Maryland University of Integrative Health Herb Dispensary EXTRACT FIXED OIL(s) SPIRITS *Price: 0.28 cents per ml* KEY: Dry=d Fresh=f Bark=b Flower=f Fruit/Seed=s Leaf=l Herb=H Rhizome=z Root=r Stem=st Resin=rsn Note: Part ratio may vary due to supply. Updated 1/29/2015 Type Botanical Part/Ratio Mfg extract Achillea millefolium df 1:3 Galen’s Way extract Aconitum carmichaeli* dr 1:10 Heron Botanicals extract Acorus calamus fr 1:1.5 Galen’s Way extract Actaea racemosa dr 1:3 Galen’s Way extract Aesculus hippocastanum ds 1:2.5 Galen’s Way extract Agathosma betulina dl 1:3 Herb Pharm extract Agrimonia eupatoria dh 1:3 Galen’s Way extract Albizia lebbek db 1:2 Medi-Herb extract Alchemilla xanthochlora dh 1:3 Galen’s Way extract Althaea officinalis dr 1:6 Galen’s Way extract Ammi visnaga ds 1:3 Galen’s Way extract Anemopsis californica** dr,z 1:3 Galen’s Way extract Angelica archangelica dr 1:3 Galen’s Way extract Angelica sinensis dr 1:2 Gaia dr 1:3 Galen’s Way extract Apium graveoloens ds 1:3 Galen’s Way extract Arctium lappa (seed) ds 1:3 Galen’s Way extract Arctium lappa (root) dr 1:3 Galen’s Way extract Artemisia absinthium dh1:5 Herb Pharm extract Artemisia annua dh 1:4 Herbalist & Alch. extract Artemisia vulgaris dh 1:3 Galen’s Way extract Asclepias tuberosa dr 1:3 Galen’s Way extract Asparagus racemosus dr 1:5 Herb-Pharm extract Astragalus membranaceus (glycerite) dr 1:4 Galen’s Way extract Avena sativa fs 1:1.25 Galen’s Way extract Bacopa monnieri dh 1:2 Medi-Herb extract Baptisia tinctoria fr 1:2-1:3 Galen’s Way extract Bryonia cretica* dr 1:10 Heron Botanicals extract Bupleurum falcatum dr 1:2 Medi-Herb extract Cactus grandiflorus fst 1:1.5 Herb Pharm extract Calendula officinalis df 1:3 Galen’s Way or df 1:5 Herb Pharm extract Capsella bursa-pastoris fh 1:1.5 Galen’s Way extract Capsicum frutescens ds 1:7 Galen’s Way extract Caulophyllum thalictroides dr 1:3 Galen’s Way extract Ceanothus americanus dr 1:3 Galen’s Way extract Centella asiatica dh 1:2 Galen’s Way extract Chamaelirium luteum dz 1:4-1:5 Herbalist & Alch. -

Proprietary Products Guide
Proprietary Formulas Our proprietary blends are unlike any on the market and offer acute relief as well as tonic support for constitutional health. Highly effective and accessibly priced, our remedies inspire repeat purchases and loyal following. Our practitioner partners love having these on hand, and find they are well matched for many common health concerns. All our formulas are available for distribution under your label and are also offered bulk. Commitment to Quality Superior products are only possible with superior medicinals. We source from the most reputable herb suppliers, including local farms and responsible wildcrafters. We are proud to work only with Chinese herb distributors who employ rigid standards and quality assurance testing, and continually offer us better access to organically grown Chinese medicinals. All of the Western herbs we buy are certified organic, locally grown, or ethically wildcrafted. Each of our vendors goes through our GMP supplier qualification program, and each herb is analyzed against our rigorous specifications to ensure the identity, purity, and potency of every medicinal. We are registered to produce certified organic products by CCOF. Our Approach Launched by a professional herbalist / acupuncturist, and a medical doctor, Five Flavors Herbs bridges the therapeutic traditions of East and West. We combine scientific research and clinical practice with millenniums old theory of healing, offering reliable tools for practitioners and purveyors. Truly unique formulations, our extract line stands out both in approach and effectiveness. Nervous System Remedies Elation (Xiao Yao San +) ~ mood support Lifts the mood and promotes a healthy emotional response to stress and monthly hormonal changes.* Take 15-60 drops in ¼ cup water 3-5 times daily or as directed. -

How Can Synergism of Traditional Medicines Benefit from Network
molecules Review How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Haidan Yuan 1,2, Qianqian Ma 1, Heying Cui 3, Guancheng Liu 1, Xiaoyan Zhao 1, Wei Li 1 and Guangchun Piao 1,2,* 1 College of Pharmacy, Yanbian University; 977 Gongyuan Street, Yanji 133002, China; [email protected] (H.Y.); [email protected] (Q.M.); [email protected] (G.L.); [email protected] (X.Z.); [email protected] (W.L.) 2 Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, Yanji 133002, China 3 College of Traditional Chinese Medicine, Yanbian University; Yanji 133002, China; [email protected] * Correspondence: [email protected]; Tel.: +86-433-243-6008 Received: 24 April 2017; Accepted: 5 July 2017; Published: 7 July 2017 Abstract: Many prescriptions of traditional medicines (TMs), whose efficacy has been tested in clinical practice, have great therapeutic value and represent an excellent resource for drug discovery. Research into single compounds of TMs, such as artemisinin from Artemisia annua L., has achieved great success; however, it has become evident that a TM prescription (which frequently contains various herbs or other components) has a synergistic effect in effecting a cure or reducing toxicity. Network pharmacology targets biological networks and analyzes the links among drugs, targets, and diseases in those networks. Comprehensive, systematic research into network pharmacology is consistent with the perspective of holisticity, which is a main characteristic of many TMs. By means of network pharmacology, research has demonstrated that many a TM show a synergistic effect by acting at different levels on multiple targets and pathways. -

An Acupuncture Prescription Suggested for Treating Primary Dysmenorrhea
Open Access Journal of Complementary & L UPINE PUBLISHERS Alternative Medicine Open Access DOI: 10.32474/OAJCAM.2018.01.000111 ISSN: 2644-1217 Review Article An Acupuncture Prescription Suggested for Treating Primary Dysmenorrhea Tong Zheng Hong* As-You-Wish Healthcare Institute, Taiwan Received: October 22, 2018; Published: October 29, 2018 *Corresponding author: Health Sciences in IL, Taiwan Tong Zheng Hong, As-You-Wish Healthcare Institute, MS in Acupuncture awarded by National University of Abstract Primary dysmenorrhea (PM) is the issue that needs attention because most of the women during reproductive age are affected with it, causing discomfort in daily life. Acupuncture has been seen as an alternative therapy and been considered “essential health benefits” in the western countries with more and more popularity, which has confirmed TCM and acupuncture effective as Western medicationsKeywords: for PM. However, all the TCM and acupuncture must follow pattern identification for the best results. Primary dysmenorrhea; NSAIDs; pattern identification Introduction It is estimated the percentage of women affected by the past 40 years and been considered “essential health dysmenorrhea ranges from 16% to 90%. Dysmenorrhea, the most prevalent gynecological issue with the women during reproductive only Chinese herbal medicine (CHM), including crude herbs, is well- benefits” in the US and in high demand in many countries [1,3]. Not age, refers to the occurrence of painful uterine cramping associated accepted and commonly used in granule and powder in Taiwan to with menses, which occurs before, during and/or after menstrual treat various health conditions, but acupuncture has gained more periods with symptoms, such as low pelvic discomfort/pain, low back pain, pain radiated to the anterior thighs, nausea, vomiting, given to women with primary dysmenorrhea [4]. -

Supporting Musculoskeletal Health Guide
Supporting Musculoskeletal Health High-quality products for your dispensary Boswellia (Boswellia serrata) California Poppy (Eschscholzia californica ) Herbal Solutions for Musculoskeletal Health MediHerb® products are developed by experts and leaders in the field of herbalism. Using the latest scientific evidence, coupled with centuries of traditional knowledge, MediHerb® brings you products that are expertly formulated and of the highest quality. A healthy musculoskeletal system is vital for the body’s normal function. Musculoskeletal discomfort can limit mobility, cause additional stress and affect quality of life. This guide features the core MediHerb® tablet and liquid formulations that support musculoskeletal health to promote vitality and well-being at all stages of life.* Turmeric (Curcuma longa) Willow Bark (Salix purpurea) 2 Table of Contents Bone Support .................................................................................................4 Bone Complex .................................................................................................4 Joint Support ..................................................................................................5 Boswellia Complex .........................................................................................5 Celery Seed 1:2 ...............................................................................................6 Ginger 1:2 ........................................................................................................6 Saligesic ............................................................................................................7 -

The Science Behind Our Formulas
Brazen The Science Behind Our Formulas © Brazen® 2019 Brazen PMS Support We started with a formula that was effective for more than 75% of women. Then we made it better. Our PMS Support blend is based on one of the most widely prescribed traditional formulas for PMS. The base formula we chose is known for its anti-anxiety and antidepressant effects, as well as its ability to address the physical symptoms of PMS like fatigue, insomnia, breast pain, anxiety, irritability, mood swings, and tension. We also wanted to make sure our formula was strong enough for even the worst PMS. In one study for PMDD, a severe disease with PMS-like symptoms, the base formula we settled on was effective for more than 75% of women, with 46.7% of women achieving remission according to the HAM-D depression rating scale. 81% of patients at the conclusion of the study no longer met the DSM-IV criteria for PMDD. That sounded like a great place to start, so we set about making our formula the best in the biz. Ingredients: Angelica Root, White Peony Root, Poria, Silk Tree Bark, Valerian Root, Siberian Milkwort Root, Bupleurum Root, Red Peony Root, Baikal Skullcap Root, Cyperus Rhizome, Bai- zhu Atractylodes Rhizome, Turmeric Tuber Root, Tree Peony Root, Mint, Licorice Root Brazen Cramp Support A tradition of formulas almost twice as effective for treating menstrual pain as pharmaceutical treatments, all without the significant adverse effects. Research shows traditional herbal medicine to be especially effective for common menstrual complaints. An industry leading Cochrane Systematic Review of 39 randomized control trials, including more than 3,400 women found that herbal formulas like ours were almost twice as effective for treating menstrual pain as pharmaceutical treatments like over the counter painkillers or birth control pills, all without significant adverse effects. -

S41438-020-00450-6.Pdf
Xu et al. Horticulture Research (2021) 8:16 Horticulture Research https://doi.org/10.1038/s41438-020-00450-6 www.nature.com/hortres ARTICLE Open Access Integration of full-length transcriptomics and targeted metabolomics to identify benzylisoquinoline alkaloid biosynthetic genes in Corydalis yanhusuo Dingqiao Xu1,HanfengLin 2,YupingTang1,LuHuang1,JianXu2,SihuiNian3 and Yucheng Zhao 2 Abstract Corydalis yanhusuo W.T. Wang is a classic herb that is frequently used in traditional Chinese medicine and is efficacious in promoting blood circulation, enhancing energy, and relieving pain. Benzylisoquinoline alkaloids (BIAs) are the main bioactive ingredients in Corydalis yanhusuo. However, few studies have investigated the BIA biosynthetic pathway in C. yanhusuo, and the biosynthetic pathway of species-specific chemicals such as tetrahydropalmatine remains unclear. We performed full-length transcriptomic and metabolomic analyses to identify candidate genes that might be involved in BIA biosynthesis and identified a total of 101 full-length transcripts and 19 metabolites involved in the BIA biosynthetic pathway. Moreover, the contents of 19 representative BIAs in C. yanhusuo were quantified by classical targeted metabolomic approaches. Their accumulation in the tuber was consistent with the expression patterns of identified BIA biosynthetic genes in tubers and leaves, which reinforces the validity and reliability of the analyses. Full- length genes with similar expression or enrichment patterns were identified, and a complete BIA biosynthesis pathway fi 1234567890():,; 1234567890():,; 1234567890():,; 1234567890():,; in C. yanhusuo was constructed according to these ndings. Phylogenetic analysis revealed a total of ten enzymes that may possess columbamine-O-methyltransferase activity, which is the final step for tetrahydropalmatine synthesis. Our results span the whole BIA biosynthetic pathway in C. -
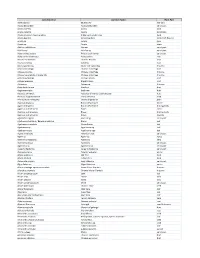
Testing Services List V2020
Latin Binomial Common Name Plant Part Abies sibirica Siberian Fir leaf (oil) Acacia Berlandieri Acacia Berlandieri aerial part Acacia catechu Acacia bark Acacia catechu Acacia gum/resin Acacia nilotica / Acacia arabica Indian gum arabic tree bark Acacia Rigidula Acacia Rigidula herb (leaf, flower) Acacia sp. Acacia gum Acacia sp. Acacia stem Achillea millefolium Yarrow aerial part Achillea sp. Achillea sp. aerial part Achyranthes aspera Prickly chaff flower aerial part Achyranthes bidentata Achyranthes root Aconite carmichaeli Chinese Aconite root Acorus calamus Calamus root Acorus gramineus Grass-leaf sweetflag rhizome Actaea cimicifuga Chinese cimicifuga root Actaea dahurica Chinese cimicifuga rhizome Actaea heracleifolia / Sheng Ma Chinese cimicifuga rhizome Actaea podocarpa Yellow Cohosh root Actaea racemosa Black Cohosh root Actaea sp. Actaea sp. rhizome Actinidia deliciosa Kiwifruit fruit Aegle marmelos Bael tree fruit Aesculus chinensis Aesculus chinensis [Sapindaceae] fruit Aesculus hippocastanum Horse chestnut seed Aframomum melegueta Grains of paradise grain Agaricus bisporus Button Mushroom entire Agaricus bisporus Button Mushroom fruiting body Agaricus subrufescens Blazei entire Agaricus subrufescens Blazei fruiting body Agaricus subrufescens Blazei mycelia Agastache rugosa Huo Xiang aerial part Agathosma betulina / Barosma betulina Buchu leaf Agathosma crenulata Ovate Buchu leaf Agathosma sp. Agathosma sp. leaf Agathosma spp. Agathosma spp. leaf Agave americana American aloe aerial part Agave sp. Agave sp. syrup Agrimonia