Quality Evaluation of Corydalis Yanhusuo by High-Performance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Network Pharmacology and Traditional Chinese Medicine: Devel- Opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1*
ISSN: 2377-3634 Lu et al. Int J Diabetes Clin Res 2017, 4:077 DOI: 10.23937/2377-3634/1410077 Volume 4 | Issue 2 International Journal of Open Access Diabetes and Clinical Research REVIEW ARTICLE Network Pharmacology and Traditional Chinese Medicine: Devel- opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1* 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China Check for 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China updates *Corresponding author: Yitao Chen, MD, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected]; Changyu Li, MD, College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected] Abstract Research and Development Dilemma for Anti- Diabetes Drugs Partly due to the failure of single-target drugs, diabetes mel- litus, a chronic metabolic disease with complex pathogene- Type 2 Diabetes Mellitus (T2DM), generally agreed sis and long-term medication requirements, is increasing in to be caused by insulin resistance and/or insulin defi- prevalence worldwide and urgently needs multi-component and multi-target treatments. Traditional Chinese herbs are ciency, constitutes almost 95 percent of all diabetes the principal drug of Chinese medicine, which is effective cases [4]. Because of the pathogenesis, the mainstre- against diabetes. However, Chinese herbs’ mechanism of am anti-diabetic drugs are insulin secretagogues (sul- action is difficult to elucidate due to its multiple components phonylureas and meglitinide analogues), insulin sensi- and multi-target effects. -

The Alchemical Body in Daoism
The Alchemical Body in Daoism FABRIZIO PREGADIO Abstract This paper surveys some of the main features of the view of the human body in Daoist internal alchemy (neidan 內丹). The first sections discuss three different terms that refer to the body; cosmological, political, theological, natural, and al- chemical metaphors used to describe it; and the use of the body as a support for the system of correspondences that tie the human being to the cosmos. On this background, the development of internal alchemy closely relates to the earlier Daoist meditation practices on the inner gods. The figure of the Red Child (the innermost deity of the human being), in particular, bears close analogies to the “embryo” that alchemists generate through their practices. The final sections are concerned with the two main alchemical charts of the human body and with the use of the Buddhist concept of “dharma-body,” which some masters describe as the true immortal body. It is virtually impossible to distinguish the Daoist understanding of the body from its understanding of the human being, and this point consti- tutes on its own a central aspect of the Daoist way of seeing. For a Daoist, knowledge of the anatomic forms and the physiological workings of the body, or any of its parts and organs, is virtually irrelevant. The physical body performs another function: it serves to support different sets of metaphors that express the relation of the whole person to the Dao, the ultimate principle to which the person owes its existence. These metaphors may be cosmological (the body as a microcosm), political (the body as an administrative system), theological (the body as the residence of inner gods), natural (the body as a “landscape”), and alchemical (the body as a laboratory for compounding the elixir), to name the most important ones. -

Supplementary Materials 1
Supplementary materials 1 Table S1 The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Botanical Pin Yin Name Form Ingredients Recommendation for daily intake (g) preparations (汉语) Plant food supplements (PFS) Si Ji Kang Mei Yang Xin Yuan -Rou Dou Kou xylooligosaccharide, isomalt, nutmeg (myristica PFS 1 Fu He Tang Pian tablet 4 tablets (1.4 g) fragrans), galangal, cinnamon, chicken gizzards (四季康美养心源-肉豆蔻复合糖片) Ai Si Meng Hui Xiang fennel seed, figs, prunes, dates, apples, St.Johns 2-4 tablets (2.8-5.6 g) PFS 2 Fu He Pian tablet Breed, jamaican ginger root (爱司盟茴香复合片) Zi Ran Mei Xiao Hui Xiaong Jiao Nang foeniculi powder, cinnamomi cortex, papaya PFS 3 capsule concentrated powder, green oat concentrated powder, 3 capsules (1.8 g) (自然美小茴香胶囊) brewer’s yeast, cabbage, monkey head mushroom An Mei Qi Hui Xiang Cao Ben Fu He Pian fennel seed, perilla seed, cassia seed, herbaceous PFS 4 tablet 1-2 tablets (1.4-2.8 g) (安美奇茴香草本复合片) complex papaya enzymes, bromelain enzymes, lactobacillus An Mei Qi Jiao Su Xian Wei Ying Yang Pian acidophilus, apple fiber, lemon plup fiber, fennel PFS 5 tablet seed, cascara sagrada, jamaican ginger root, herbal 2 tablets (2.7 g) (安美奇酵素纤维营养片) support complex (figs, prunes, dates, apples, St. Johns bread) Table S1 (continued) The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Pin Yin Name Botanical Form Ingredients Recommendation for daily intake (g) preparations (汉语) Gan Cao Pian glycyrrhiza uralensis, licorice -

Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 1-18-2012 Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats Zhiying Dou Tianjin University of Traditional Chinese Medicine Kefeng Li Michigan Technological University Ping Wang Tianjin University of Traditional Chinese Medicine Liu Cao Tianjin University of Traditional Chinese Medicine Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Recommended Citation Dou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules, 17(1), 951-970. http://doi.org/10.3390/molecules17010951 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1969 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Molecules 2012, 17, 951-970; doi:10.3390/molecules17010951 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats Zhiying Dou 1,*, Kefeng Li 2, Ping Wang 1 and Liu Cao 1 1 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China 2 Department of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-22-5959-6235. Received: 29 November 2011; in revised form: 5 January 2012 / Accepted: 9 January 2012 / Published: 18 January 2012 Abstract: Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. -

Qi Gong and High Blood Pressure, Part II
Qi Gong and High Blood Pressure, Part II Published in New Health Digest, February 2007 issue Traditional Chinese Medicine looks at hypertension (high blood pressure) as being related to Liver and Heart energy imbalances and stagnation. Diet and exercise can go a long way toward alleviating these issues. In last month’s article I discussed diet changes that could be made to reduce stagnation and cleanse and improve the cardiovascular system. This article will cover the specific Qi Gong techniques that work best to help with this. There are two very effective exercises, the Liver and Heart Sounds, from an ancient form of Qi Gong called the Six Healing Sounds. These exercises date back to the 7th century B.C. and improve our health by releasing out stagnant energy as well as stuck emotions from our bodies. Do the Liver Sound first as many times in a row as feels comfortable and then do the Heart Sound next for as many times as feels good. Repeat these two exercises three times per day. Liver Sound Sit on the edge of a chair or your bed. Place your palms facing up on your lap, elbows out slightly and away from your body. Keep your back straight and relaxed and your chin in slightly. You can have your eyes closed or opened slightly. Begin the posture by bringing your hands out from the sides of the body. Liver Sound Stretch them out as far as they will go while keeping the elbows bent “Tshhh” slightly and the shoulders relaxed. Continue to raise the hands up until they (lean to left) meet over the head. -

NRDC: Generally Recognized As Secret
NRDC Report April 2014 Generally Recognized as Secret: Chemicals Added to Food in the United States Tom Neltner, J.D., Maricel Maffini, Ph.D. Natural Resources Defense Council In April 2014, the Natural Resources Defense Council (NRDC) released a report raising concerns about a loophole in the Food Additives Amendment of 1958 for substances designated by food manufacturers as “generally recognized as safe” (GRAS). The report identified 56 companies that appeared to market 275 chemicals for use in food based on undisclosed GRAS safety determinations. For each chemical we identified in this study, we did not find evidence that FDA had cleared them for use in food. The 1958 law exempted from the formal, extended FDA approval process common food ingredients like vinegar and vegetable oil whose use qualifies as GRAS. It may have appeared reasonable at the time, but that exemption has been stretched into a loophole that has swallowed the law. The exemption allows manufacturers to make safety determinations that the uses of their newest chemicals in food are safe without notifying the FDA. The agency’s attempts to limit these undisclosed GRAS determinations by asking industry to voluntarily inform the FDA about their chemicals are insufficient to ensure the safety of our food in today’s global marketplace with a complex food supply. Furthermore, no other developed country in the world has a system like GRAS to provide oversight of food ingredients. In Table 1 and 2 of the report, NRDC identified the 56 companies and the number of chemicals that each company appeared to market as GRAS without FDA clearance. -

A Systematic Review of the Effectiveness of Qigong Exercise in Cardiac Rehabilitation
The American Journal of Chinese Medicine, Vol. 40, No. 2, 255–267 © 2012 World Scientific Publishing Company Institute for Advanced Research in Asian Science and Medicine DOI: 10.1142/S0192415X12500206 A Systematic Review of the Effectiveness of Qigong Exercise in Cardiac Rehabilitation Cecilia Lai-Wan Chan,* Chong-Wen Wang,* Rainbow Tin-Hung Ho,* Andy Hau-Yan Ho,* Eric Tat-Chi Ziea,† Vivian Chi-Woon Taam Wong† and Siu-Man Ng* *Centre on Behavioral Health University of Hong Kong, HKSAR, China †The Chinese Medicine Department Hospital Authority, HKSAR, China Abstract: The objective of this study was to assess evidence for the efficacy and effectiveness of Chinese qigong exercise in rehabilitative programs among cardiac patients. Thirteen databases were searched through to November 2010, and all controlled clinical trials on Chinese qigong exercise among patients with chronic heart diseases were included. For each included study, data was extracted and validity was assessed. Study quality was evaluated and summarized using both the Jadad Scale and the criteria for levels of evidence. Seven randomized controlled trials (RCTs) and one non-randomized controlled clinical trial (CCT) published between 1988 and 2007 met the inclusion criteria. In total, these studies covered 540 patients with various chronic heart diseases including atrial fibrillation, coronary artery disease, myocardial infarct, valve replacement, and ischemic heart disease. Outcome by BOSTON UNIVERSITY on 10/26/14. For personal use only. measures emerged in these studies included subjective outcomes such as symptoms and quality of life; and objective outcomes such as blood pressure, ECG findings, and exercise Am. J. Chin. Med. 2012.40:255-267. -
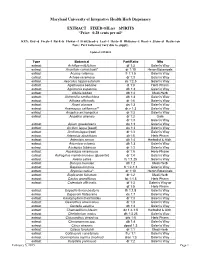
SPIRITS *Price: 0.28 Cents Per Ml*
Maryland University of Integrative Health Herb Dispensary EXTRACT FIXED OIL(s) SPIRITS *Price: 0.28 cents per ml* KEY: Dry=d Fresh=f Bark=b Flower=f Fruit/Seed=s Leaf=l Herb=H Rhizome=z Root=r Stem=st Resin=rsn Note: Part ratio may vary due to supply. Updated 1/29/2015 Type Botanical Part/Ratio Mfg extract Achillea millefolium df 1:3 Galen’s Way extract Aconitum carmichaeli* dr 1:10 Heron Botanicals extract Acorus calamus fr 1:1.5 Galen’s Way extract Actaea racemosa dr 1:3 Galen’s Way extract Aesculus hippocastanum ds 1:2.5 Galen’s Way extract Agathosma betulina dl 1:3 Herb Pharm extract Agrimonia eupatoria dh 1:3 Galen’s Way extract Albizia lebbek db 1:2 Medi-Herb extract Alchemilla xanthochlora dh 1:3 Galen’s Way extract Althaea officinalis dr 1:6 Galen’s Way extract Ammi visnaga ds 1:3 Galen’s Way extract Anemopsis californica** dr,z 1:3 Galen’s Way extract Angelica archangelica dr 1:3 Galen’s Way extract Angelica sinensis dr 1:2 Gaia dr 1:3 Galen’s Way extract Apium graveoloens ds 1:3 Galen’s Way extract Arctium lappa (seed) ds 1:3 Galen’s Way extract Arctium lappa (root) dr 1:3 Galen’s Way extract Artemisia absinthium dh1:5 Herb Pharm extract Artemisia annua dh 1:4 Herbalist & Alch. extract Artemisia vulgaris dh 1:3 Galen’s Way extract Asclepias tuberosa dr 1:3 Galen’s Way extract Asparagus racemosus dr 1:5 Herb-Pharm extract Astragalus membranaceus (glycerite) dr 1:4 Galen’s Way extract Avena sativa fs 1:1.25 Galen’s Way extract Bacopa monnieri dh 1:2 Medi-Herb extract Baptisia tinctoria fr 1:2-1:3 Galen’s Way extract Bryonia cretica* dr 1:10 Heron Botanicals extract Bupleurum falcatum dr 1:2 Medi-Herb extract Cactus grandiflorus fst 1:1.5 Herb Pharm extract Calendula officinalis df 1:3 Galen’s Way or df 1:5 Herb Pharm extract Capsella bursa-pastoris fh 1:1.5 Galen’s Way extract Capsicum frutescens ds 1:7 Galen’s Way extract Caulophyllum thalictroides dr 1:3 Galen’s Way extract Ceanothus americanus dr 1:3 Galen’s Way extract Centella asiatica dh 1:2 Galen’s Way extract Chamaelirium luteum dz 1:4-1:5 Herbalist & Alch. -

Neidan Qigong
Neidan Qigong Neidan qigong means “inner elixir energy practice”. This form purifies and tonifies the three dantian and circulates qi to nourish the natural process of internal alchemy. This practice should be coupled with daily zuowang meditation (sitting & forgetting) and a healthy, moderate lifestyle including a balanced diet, regular sleep, and not too much stress or agitation. Relax and trust the natural process… Purging and Cleansing Stand comfortably, feet under hips or together, arms, legs, and spine relaxed, breathing naturally into the belly, arms relaxed to sides, mind empty and calm. Let the tongue rest against the upper palate. Just stand like this for awhile until you feel settled and ready to proceed. Inhale, letting arms float up to sides, palms up, feeling qi rise up the back, until arms are above head, palms facing one another, embracing the qi of heaven. Exhale, dropping elbows, bring arms down in front of body, palms facing inward/down toward upper, then middle, then lower dantian and/or spine and internal organs, sending qi inward to bathe the dantian, organs, blood, marrow, specific injured areas or the entire body, letting everything settle downward, until arms rest down at sides. Repeat 3, 6, or 9 times Gathering Qi into the Dantian Inhale, drawing hands inward to touch lower belly, gathering the qi of earth into the lower dantian. You can visualize earth qi entering from below (legs, perineum) or cosmic qi entering from 10 directions into lower dantian, forming a single point of light. Natural, not forced, just like breathing air or swallowing food. -

Proprietary Products Guide
Proprietary Formulas Our proprietary blends are unlike any on the market and offer acute relief as well as tonic support for constitutional health. Highly effective and accessibly priced, our remedies inspire repeat purchases and loyal following. Our practitioner partners love having these on hand, and find they are well matched for many common health concerns. All our formulas are available for distribution under your label and are also offered bulk. Commitment to Quality Superior products are only possible with superior medicinals. We source from the most reputable herb suppliers, including local farms and responsible wildcrafters. We are proud to work only with Chinese herb distributors who employ rigid standards and quality assurance testing, and continually offer us better access to organically grown Chinese medicinals. All of the Western herbs we buy are certified organic, locally grown, or ethically wildcrafted. Each of our vendors goes through our GMP supplier qualification program, and each herb is analyzed against our rigorous specifications to ensure the identity, purity, and potency of every medicinal. We are registered to produce certified organic products by CCOF. Our Approach Launched by a professional herbalist / acupuncturist, and a medical doctor, Five Flavors Herbs bridges the therapeutic traditions of East and West. We combine scientific research and clinical practice with millenniums old theory of healing, offering reliable tools for practitioners and purveyors. Truly unique formulations, our extract line stands out both in approach and effectiveness. Nervous System Remedies Elation (Xiao Yao San +) ~ mood support Lifts the mood and promotes a healthy emotional response to stress and monthly hormonal changes.* Take 15-60 drops in ¼ cup water 3-5 times daily or as directed. -

How Can Synergism of Traditional Medicines Benefit from Network
molecules Review How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Haidan Yuan 1,2, Qianqian Ma 1, Heying Cui 3, Guancheng Liu 1, Xiaoyan Zhao 1, Wei Li 1 and Guangchun Piao 1,2,* 1 College of Pharmacy, Yanbian University; 977 Gongyuan Street, Yanji 133002, China; [email protected] (H.Y.); [email protected] (Q.M.); [email protected] (G.L.); [email protected] (X.Z.); [email protected] (W.L.) 2 Key Laboratory of Natural Resources of Changbai Mountain & Functional Molecules, Yanbian University, Ministry of Education, Yanji 133002, China 3 College of Traditional Chinese Medicine, Yanbian University; Yanji 133002, China; [email protected] * Correspondence: [email protected]; Tel.: +86-433-243-6008 Received: 24 April 2017; Accepted: 5 July 2017; Published: 7 July 2017 Abstract: Many prescriptions of traditional medicines (TMs), whose efficacy has been tested in clinical practice, have great therapeutic value and represent an excellent resource for drug discovery. Research into single compounds of TMs, such as artemisinin from Artemisia annua L., has achieved great success; however, it has become evident that a TM prescription (which frequently contains various herbs or other components) has a synergistic effect in effecting a cure or reducing toxicity. Network pharmacology targets biological networks and analyzes the links among drugs, targets, and diseases in those networks. Comprehensive, systematic research into network pharmacology is consistent with the perspective of holisticity, which is a main characteristic of many TMs. By means of network pharmacology, research has demonstrated that many a TM show a synergistic effect by acting at different levels on multiple targets and pathways. -

The Relationship Between the Kidney and the Heart in Chinese Medicine - Part Two
1 © Chinese Medicine Times - 2012 The Relationship between the Kidney and the Heart in Chinese Medicine - Part Two by Leon Hammer n part one, we observed that from the very it is familiar to all. `Heart Yang’ fashions the beginning in-utero and throughout life the primacy imagination and inspiration of `Heart Yin’ into a I of the heart, and especially of the water-fire recognizable object that we recognize and appreciate as relationship [Kidney Heart Harmony]. a work of art or a discovery of science. It is what Thomas Edison called `perspiration’. The ultimate Kidney-Heart Disharmony and Sleep source is Kidney Essence. `Pericardium Yin’ protects creation and `Pericardium Yang’ projects it, enhanced harmoniously by the Triple Burner. If both the Heart and Kidney are yin deficient [left distal and proximal positions are Tight, sleep is very All of the functions of the Water from Divine Love and restless. One will easily wake at the smallest sound or Divine Spirit and Divine Power, awe and faith, will disturbance. and courage in the face of the unknown, all are potential becoming a reality through the expressive If the Heart is yin deficient [left distal Tight] and power of the Heart, the great communicator that Kidney Qi-Yang is deficient [left proximal position reaches out to the world with the `Word’. Will power, Feeble-Absent], sleep resembles the Heart Blood zhi, a prime function associated with the Kidney, has deficient pattern. One wakes after approximately five been described as the ability to put one’s Heart into hours sleep and then can return to sleep after a short what one is doing.