Isoquinoline Alkaloids Isolated from Corydalis Yanhusuo and Their Binding Affinities at the Dopamine D1 Receptor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Network Pharmacology and Traditional Chinese Medicine: Devel- Opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1*
ISSN: 2377-3634 Lu et al. Int J Diabetes Clin Res 2017, 4:077 DOI: 10.23937/2377-3634/1410077 Volume 4 | Issue 2 International Journal of Open Access Diabetes and Clinical Research REVIEW ARTICLE Network Pharmacology and Traditional Chinese Medicine: Devel- opment of Anti-Diabetic Therapies Zhongxia Lu1, Wenjun Xu1, Xi Chen2, Changyu Li3* and Yitao Chen1* 1College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China 2College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, China Check for 3College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China updates *Corresponding author: Yitao Chen, MD, College of Life Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected]; Changyu Li, MD, College of Pharmacy, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, 310053, China, E-mail: [email protected] Abstract Research and Development Dilemma for Anti- Diabetes Drugs Partly due to the failure of single-target drugs, diabetes mel- litus, a chronic metabolic disease with complex pathogene- Type 2 Diabetes Mellitus (T2DM), generally agreed sis and long-term medication requirements, is increasing in to be caused by insulin resistance and/or insulin defi- prevalence worldwide and urgently needs multi-component and multi-target treatments. Traditional Chinese herbs are ciency, constitutes almost 95 percent of all diabetes the principal drug of Chinese medicine, which is effective cases [4]. Because of the pathogenesis, the mainstre- against diabetes. However, Chinese herbs’ mechanism of am anti-diabetic drugs are insulin secretagogues (sul- action is difficult to elucidate due to its multiple components phonylureas and meglitinide analogues), insulin sensi- and multi-target effects. -

The Alchemical Body in Daoism
The Alchemical Body in Daoism FABRIZIO PREGADIO Abstract This paper surveys some of the main features of the view of the human body in Daoist internal alchemy (neidan 內丹). The first sections discuss three different terms that refer to the body; cosmological, political, theological, natural, and al- chemical metaphors used to describe it; and the use of the body as a support for the system of correspondences that tie the human being to the cosmos. On this background, the development of internal alchemy closely relates to the earlier Daoist meditation practices on the inner gods. The figure of the Red Child (the innermost deity of the human being), in particular, bears close analogies to the “embryo” that alchemists generate through their practices. The final sections are concerned with the two main alchemical charts of the human body and with the use of the Buddhist concept of “dharma-body,” which some masters describe as the true immortal body. It is virtually impossible to distinguish the Daoist understanding of the body from its understanding of the human being, and this point consti- tutes on its own a central aspect of the Daoist way of seeing. For a Daoist, knowledge of the anatomic forms and the physiological workings of the body, or any of its parts and organs, is virtually irrelevant. The physical body performs another function: it serves to support different sets of metaphors that express the relation of the whole person to the Dao, the ultimate principle to which the person owes its existence. These metaphors may be cosmological (the body as a microcosm), political (the body as an administrative system), theological (the body as the residence of inner gods), natural (the body as a “landscape”), and alchemical (the body as a laboratory for compounding the elixir), to name the most important ones. -

Supplementary Materials 1
Supplementary materials 1 Table S1 The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Botanical Pin Yin Name Form Ingredients Recommendation for daily intake (g) preparations (汉语) Plant food supplements (PFS) Si Ji Kang Mei Yang Xin Yuan -Rou Dou Kou xylooligosaccharide, isomalt, nutmeg (myristica PFS 1 Fu He Tang Pian tablet 4 tablets (1.4 g) fragrans), galangal, cinnamon, chicken gizzards (四季康美养心源-肉豆蔻复合糖片) Ai Si Meng Hui Xiang fennel seed, figs, prunes, dates, apples, St.Johns 2-4 tablets (2.8-5.6 g) PFS 2 Fu He Pian tablet Breed, jamaican ginger root (爱司盟茴香复合片) Zi Ran Mei Xiao Hui Xiaong Jiao Nang foeniculi powder, cinnamomi cortex, papaya PFS 3 capsule concentrated powder, green oat concentrated powder, 3 capsules (1.8 g) (自然美小茴香胶囊) brewer’s yeast, cabbage, monkey head mushroom An Mei Qi Hui Xiang Cao Ben Fu He Pian fennel seed, perilla seed, cassia seed, herbaceous PFS 4 tablet 1-2 tablets (1.4-2.8 g) (安美奇茴香草本复合片) complex papaya enzymes, bromelain enzymes, lactobacillus An Mei Qi Jiao Su Xian Wei Ying Yang Pian acidophilus, apple fiber, lemon plup fiber, fennel PFS 5 tablet seed, cascara sagrada, jamaican ginger root, herbal 2 tablets (2.7 g) (安美奇酵素纤维营养片) support complex (figs, prunes, dates, apples, St. Johns bread) Table S1 (continued) The characteristics of botanical preparations potentially containing alkenylbenzenes on the Chinese market. Pin Yin Name Botanical Form Ingredients Recommendation for daily intake (g) preparations (汉语) Gan Cao Pian glycyrrhiza uralensis, licorice -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats
Michigan Technological University Digital Commons @ Michigan Tech Michigan Tech Publications 1-18-2012 Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats Zhiying Dou Tianjin University of Traditional Chinese Medicine Kefeng Li Michigan Technological University Ping Wang Tianjin University of Traditional Chinese Medicine Liu Cao Tianjin University of Traditional Chinese Medicine Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Recommended Citation Dou, Z., Li, K., Wang, P., & Cao, L. (2012). Effect of wine and vinegar processing of Rhizoma Corydalis on the tissue distribution of tetrahydropalmatine, protopine and dehydrocorydaline in rats. Molecules, 17(1), 951-970. http://doi.org/10.3390/molecules17010951 Retrieved from: https://digitalcommons.mtu.edu/michigantech-p/1969 Follow this and additional works at: https://digitalcommons.mtu.edu/michigantech-p Part of the Biology Commons Molecules 2012, 17, 951-970; doi:10.3390/molecules17010951 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats Zhiying Dou 1,*, Kefeng Li 2, Ping Wang 1 and Liu Cao 1 1 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China 2 Department of Biological Sciences, Michigan Technological University, Houghton, MI 49931, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-22-5959-6235. Received: 29 November 2011; in revised form: 5 January 2012 / Accepted: 9 January 2012 / Published: 18 January 2012 Abstract: Vinegar and wine processing of medicinal plants are two traditional pharmaceutical techniques which have been used for thousands of years in China. -

Qi Gong and High Blood Pressure, Part II
Qi Gong and High Blood Pressure, Part II Published in New Health Digest, February 2007 issue Traditional Chinese Medicine looks at hypertension (high blood pressure) as being related to Liver and Heart energy imbalances and stagnation. Diet and exercise can go a long way toward alleviating these issues. In last month’s article I discussed diet changes that could be made to reduce stagnation and cleanse and improve the cardiovascular system. This article will cover the specific Qi Gong techniques that work best to help with this. There are two very effective exercises, the Liver and Heart Sounds, from an ancient form of Qi Gong called the Six Healing Sounds. These exercises date back to the 7th century B.C. and improve our health by releasing out stagnant energy as well as stuck emotions from our bodies. Do the Liver Sound first as many times in a row as feels comfortable and then do the Heart Sound next for as many times as feels good. Repeat these two exercises three times per day. Liver Sound Sit on the edge of a chair or your bed. Place your palms facing up on your lap, elbows out slightly and away from your body. Keep your back straight and relaxed and your chin in slightly. You can have your eyes closed or opened slightly. Begin the posture by bringing your hands out from the sides of the body. Liver Sound Stretch them out as far as they will go while keeping the elbows bent “Tshhh” slightly and the shoulders relaxed. Continue to raise the hands up until they (lean to left) meet over the head. -

L-Tetrahydropalmatine Reduces Nicotine Self-Administration and Reinstatement in Rats
Faison et al. BMC Pharmacology and Toxicology (2016) 17:49 DOI 10.1186/s40360-016-0093-6 RESEARCH ARTICLE Open Access l-tetrahydropalmatine reduces nicotine self- administration and reinstatement in rats Shamia L. Faison1,2, Charles W. Schindler2, Steven R. Goldberg2ˆ and Jia Bei Wang1* Abstract Background: The negative consequences of nicotine use are well known and documented, however, abstaining from nicotine use and achieving abstinence poses a major challenge for the majority of nicotine users trying to quit. l-Tetrahydropalmatine (l-THP), a compound extracted from the Chinese herb Corydalis, displayed utility in the treatment of cocaine and heroin addiction via reduction of drug-intake and relapse. The present study examined the effects of l-THP on abuse-related effects of nicotine. Methods: Self-administration and reinstatement testing was conducted. Rats trained to self-administer nicotine (0.03 mg/kg/injection) under a fixed-ratio 5 schedule (FR5) of reinforcement were pretreated with l-THP (3 or 5 mg/kg), varenicline (1 mg/kg), bupropion (40 mg/kg), or saline before daily 2-h sessions. Locomotor, food, and microdialysis assays were also conducted in separate rats. Results: l-THP significantly reduced nicotine self-administration (SA). l-THP’s effect was more pronounced than the effect of varenicline and similar to the effect of bupropion. In reinstatement testing, animals were pretreated with the same compounds, challenged with nicotine (0.3 mg/kg, s.c.), and reintroduced to pre-extinction conditions. l-THP blocked reinstatement of nicotine seeking more effectively than either varenicline or bupropion. Locomotor data revealed that therapeutic doses of l-THP had no inhibitory effects on ambulatory ability and that l-THP (3 and 5 mg/kg) significantly blocked nicotine induced hyperactivity when administered before nicotine. -

NRDC: Generally Recognized As Secret
NRDC Report April 2014 Generally Recognized as Secret: Chemicals Added to Food in the United States Tom Neltner, J.D., Maricel Maffini, Ph.D. Natural Resources Defense Council In April 2014, the Natural Resources Defense Council (NRDC) released a report raising concerns about a loophole in the Food Additives Amendment of 1958 for substances designated by food manufacturers as “generally recognized as safe” (GRAS). The report identified 56 companies that appeared to market 275 chemicals for use in food based on undisclosed GRAS safety determinations. For each chemical we identified in this study, we did not find evidence that FDA had cleared them for use in food. The 1958 law exempted from the formal, extended FDA approval process common food ingredients like vinegar and vegetable oil whose use qualifies as GRAS. It may have appeared reasonable at the time, but that exemption has been stretched into a loophole that has swallowed the law. The exemption allows manufacturers to make safety determinations that the uses of their newest chemicals in food are safe without notifying the FDA. The agency’s attempts to limit these undisclosed GRAS determinations by asking industry to voluntarily inform the FDA about their chemicals are insufficient to ensure the safety of our food in today’s global marketplace with a complex food supply. Furthermore, no other developed country in the world has a system like GRAS to provide oversight of food ingredients. In Table 1 and 2 of the report, NRDC identified the 56 companies and the number of chemicals that each company appeared to market as GRAS without FDA clearance. -

Up-Regulation on Cytochromes P450 in Rat Mediated by Total Alkaloid Extract from Corydalis Yanhusuo
Yan et al. BMC Complementary and Alternative Medicine 2014, 14:306 http://www.biomedcentral.com/1472-6882/14/306 RESEARCH ARTICLE Open Access Up-regulation on cytochromes P450 in rat mediated by total alkaloid extract from Corydalis yanhusuo Jingjing Yan1, Xin He1,2*, Shan Feng1, Yiran Zhai1, Yetao Ma1, Sheng Liang1 and Chunhuan Jin1 Abstract Background: Yanhusuo (Corydalis yanhusuo W.T. Wang; YHS), is a well-known traditional Chinese herbal medicine, has been used in China for treating pain including chest pain, epigastric pain, and dysmenorrhea. Its alkaloid ingredients including tetrahydropalmatine are reported to inhibit cytochromes P450 (CYPs) activity in vitro. The present study is aimed to assess the potential of total alkaloid extract (TAE) from YHS to effect the activity and mRNA levels of five cytochromes P450 (CYPs) in rat. Methods: Rats were administered TAE from YHS (0, 6, 30, and 150 mg/kg, daily) for 14 days, alanine aminotransferase (ALT) levels in serum were assayed, and hematoxylin and eosin-stained sections of the liver were prepared for light microscopy. The effects of TAE on five CYPs activity and mRNA levels were quantitated by cocktail probe drugs using a rapid chromatography/tandem mass spectrometry (LC-MS/MS) method and reverse transcription-polymerase chain reaction (RT-PCR), respectively. Results: In general, serum ALT levels showed no significant changes, and the histopathology appeared largely normal compared with that in the control rats. At 30 and 150 mg/kg TAE dosages, an increase in liver CYP2E1 and CYP3A1 enzyme activity were observed. Moreover, the mRNA levels of CYP2E1 and CYP3A1 in the rat liver, lung, and intestine were significantly up-regulated with TAE from 6 and 30 mg/kg, respectively. -

A Systematic Review of the Effectiveness of Qigong Exercise in Cardiac Rehabilitation
The American Journal of Chinese Medicine, Vol. 40, No. 2, 255–267 © 2012 World Scientific Publishing Company Institute for Advanced Research in Asian Science and Medicine DOI: 10.1142/S0192415X12500206 A Systematic Review of the Effectiveness of Qigong Exercise in Cardiac Rehabilitation Cecilia Lai-Wan Chan,* Chong-Wen Wang,* Rainbow Tin-Hung Ho,* Andy Hau-Yan Ho,* Eric Tat-Chi Ziea,† Vivian Chi-Woon Taam Wong† and Siu-Man Ng* *Centre on Behavioral Health University of Hong Kong, HKSAR, China †The Chinese Medicine Department Hospital Authority, HKSAR, China Abstract: The objective of this study was to assess evidence for the efficacy and effectiveness of Chinese qigong exercise in rehabilitative programs among cardiac patients. Thirteen databases were searched through to November 2010, and all controlled clinical trials on Chinese qigong exercise among patients with chronic heart diseases were included. For each included study, data was extracted and validity was assessed. Study quality was evaluated and summarized using both the Jadad Scale and the criteria for levels of evidence. Seven randomized controlled trials (RCTs) and one non-randomized controlled clinical trial (CCT) published between 1988 and 2007 met the inclusion criteria. In total, these studies covered 540 patients with various chronic heart diseases including atrial fibrillation, coronary artery disease, myocardial infarct, valve replacement, and ischemic heart disease. Outcome by BOSTON UNIVERSITY on 10/26/14. For personal use only. measures emerged in these studies included subjective outcomes such as symptoms and quality of life; and objective outcomes such as blood pressure, ECG findings, and exercise Am. J. Chin. Med. 2012.40:255-267. -
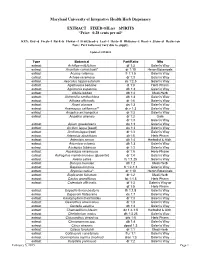
SPIRITS *Price: 0.28 Cents Per Ml*
Maryland University of Integrative Health Herb Dispensary EXTRACT FIXED OIL(s) SPIRITS *Price: 0.28 cents per ml* KEY: Dry=d Fresh=f Bark=b Flower=f Fruit/Seed=s Leaf=l Herb=H Rhizome=z Root=r Stem=st Resin=rsn Note: Part ratio may vary due to supply. Updated 1/29/2015 Type Botanical Part/Ratio Mfg extract Achillea millefolium df 1:3 Galen’s Way extract Aconitum carmichaeli* dr 1:10 Heron Botanicals extract Acorus calamus fr 1:1.5 Galen’s Way extract Actaea racemosa dr 1:3 Galen’s Way extract Aesculus hippocastanum ds 1:2.5 Galen’s Way extract Agathosma betulina dl 1:3 Herb Pharm extract Agrimonia eupatoria dh 1:3 Galen’s Way extract Albizia lebbek db 1:2 Medi-Herb extract Alchemilla xanthochlora dh 1:3 Galen’s Way extract Althaea officinalis dr 1:6 Galen’s Way extract Ammi visnaga ds 1:3 Galen’s Way extract Anemopsis californica** dr,z 1:3 Galen’s Way extract Angelica archangelica dr 1:3 Galen’s Way extract Angelica sinensis dr 1:2 Gaia dr 1:3 Galen’s Way extract Apium graveoloens ds 1:3 Galen’s Way extract Arctium lappa (seed) ds 1:3 Galen’s Way extract Arctium lappa (root) dr 1:3 Galen’s Way extract Artemisia absinthium dh1:5 Herb Pharm extract Artemisia annua dh 1:4 Herbalist & Alch. extract Artemisia vulgaris dh 1:3 Galen’s Way extract Asclepias tuberosa dr 1:3 Galen’s Way extract Asparagus racemosus dr 1:5 Herb-Pharm extract Astragalus membranaceus (glycerite) dr 1:4 Galen’s Way extract Avena sativa fs 1:1.25 Galen’s Way extract Bacopa monnieri dh 1:2 Medi-Herb extract Baptisia tinctoria fr 1:2-1:3 Galen’s Way extract Bryonia cretica* dr 1:10 Heron Botanicals extract Bupleurum falcatum dr 1:2 Medi-Herb extract Cactus grandiflorus fst 1:1.5 Herb Pharm extract Calendula officinalis df 1:3 Galen’s Way or df 1:5 Herb Pharm extract Capsella bursa-pastoris fh 1:1.5 Galen’s Way extract Capsicum frutescens ds 1:7 Galen’s Way extract Caulophyllum thalictroides dr 1:3 Galen’s Way extract Ceanothus americanus dr 1:3 Galen’s Way extract Centella asiatica dh 1:2 Galen’s Way extract Chamaelirium luteum dz 1:4-1:5 Herbalist & Alch. -

Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders
Int. J. Biol. Sci. 2018, Vol. 14 341 Ivyspring International Publisher International Journal of Biological Sciences 2018; 14(3): 341-357. doi: 10.7150/ijbs.23247 Review Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders Ghulam Hussain1,3, Azhar Rasul4,5, Haseeb Anwar3, Nimra Aziz3, Aroona Razzaq3, Wei Wei1,2, Muhammad Ali4, Jiang Li2, Xiaomeng Li1 1. The Key Laboratory of Molecular Epigenetics of MOE, Institute of Genetics and Cytology, Northeast Normal University, Changchun 130024, China 2. Dental Hospital, Jilin University, Changchun 130021, China 3. Department of Physiology, Faculty of Life Sciences, Government College University, Faisalabad, 38000 Pakistan 4. Department of Zoology, Faculty of Life Sciences, Government College University, Faisalabad, 38000 Pakistan 5. Chemical Biology Research Group, RIKEN Center for Sustainable Resource Science. 2-1 Hirosawa, Wako, Saitama 351-0198 Japan Corresponding authors: Professor Xiaomeng Li, The Key Laboratory of Molecular Epigenetics of Ministry of Education, Institute of Genetics and Cytology, Northeast Normal University, 5268 People's Street, Changchun, Jilin 130024, P.R. China. E-mail: [email protected] Tel: +86 186 86531019; Fax: +86 431 85579335 or Professor Jiang Li, Department of Prosthodontics, Dental Hospital, Jilin University, 1500 Tsinghua Road, Changchun, Jilin 130021, P.R. China. E-mail: [email protected] © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2017.10.09; Accepted: 2017.12.18; Published: 2018.03.09 Abstract Neurodegenerative diseases are conventionally demarcated as disorders with selective loss of neurons.