BIOEN 326 2013 Estimating Young's Modulus from Bond Energies And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

The Effect of Yield Strength on Inelastic Buckling of Reinforcing
Mechanics and Mechanical Engineering Vol. 14, No. 2 (2010) 247{255 ⃝c Technical University of Lodz The Effect of Yield Strength on Inelastic Buckling of Reinforcing Bars Jacek Korentz Institute of Civil Engineering University of Zielona G´ora Licealna 9, 65{417 Zielona G´ora, Poland Received (13 June 2010) Revised (15 July 2010) Accepted (25 July 2010) The paper presents the results of numerical analyses of inelastic buckling of reinforcing bars of various geometrical parameters, made of steel of various values of yield strength. The results of the calculations demonstrate that the yield strength of the steel the bars are made of influences considerably the equilibrium path of the compressed bars within the range of postyielding deformations Comparative diagrams of structural behaviour (loading paths) of thin{walled sec- tions under investigation for different strain rates are presented. Some conclusions and remarks concerning the strain rate influence are derived. Keywords: Reinforcing bars, inelastic buckling, yield strength, tensil strength 1. Introduction The impact of some exceptional loads, e.g. seismic loads, on a structure may re- sult in the occurrence of post{critical states. Therefore the National Standards regulations for designing reinforced structures on seismically active areas e.g. EC8 [15] require the ductility of a structure to be examined on a cross{sectional level, and additionally, the structures should demonstrate a suitable level of global duc- tility. The results of the examinations of members of reinforced concrete structures show that inelastic buckling of longitudinal reinforcement bars occurs in the state of post{critical deformations, [1, 2, 4, 7], and in some cases it occurs yet within the range of elastic deformations [8]. -

Lecture 42: Failure Analysis – Buckling of Columns Joshua Pribe Fall 2019 Lecture Book: Ch
ME 323 – Mechanics of Materials Lecture 42: Failure analysis – Buckling of columns Joshua Pribe Fall 2019 Lecture Book: Ch. 18 Stability and equilibrium What happens if we are in a state of unstable equilibrium? Stable Neutral Unstable 2 Buckling experiment There is a critical stress at which buckling occurs depending on the material and the geometry How do the material properties and geometric parameters influence the buckling stress? 3 Euler buckling equation Consider static equilibrium of the buckled pinned-pinned column 4 Euler buckling equation We have a differential equation for the deflection with BCs at the pins: d 2v EI+= Pv( x ) 0 v(0)== 0and v ( L ) 0 dx2 The solution is: P P A = 0 v(s x) = Aco x+ Bsin x with EI EI PP Bsin L= 0 L = n , n = 1, 2, 3, ... EI EI 5 Effect of boundary conditions Critical load and critical stress for buckling: EI EA P = 22= cr L2 2 e (Legr ) 2 E cr = 2 (Lreg) I r = Pinned- Pinned- Fixed- where g Fixed- A pinned fixed fixed is the “radius of gyration” free LLe = LLe = 0.7 LLe = 0.5 LLe = 2 6 Modifications to Euler buckling theory Euler buckling equation: works well for slender rods Needs to be modified for smaller “slenderness ratios” (where the critical stress for Euler buckling is at least half the yield strength) 7 Summary L 2 E Critical slenderness ratio: e = r 0.5 gYc Euler buckling (high slenderness ratio): LL 2 E EI If ee : = or P = 2 rr cr 2 cr L2 gg c (Lreg) e Johnson bucklingI (low slenderness ratio): r = 2 g Lr LLeeA ( eg) If : =−1 rr cr2 Y gg c 2 Lr ( eg)c with radius of gyration 8 Summary Effective length from the boundary conditions: Pinned- Pinned- Fixed- LL= LL= 0.7 FixedLL=- 0.5 pinned fixed e fixede e LL= 2 free e 9 Example 18.1 Determine the critical buckling load Pcr of a steel pipe column that has a length of L with a tubular cross section of inner radius ri and thickness t. -
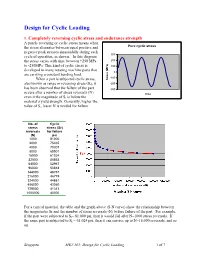
Design for Cyclic Loading, Soderberg, Goodman and Modified Goodman's Equation
Design for Cyclic Loading 1. Completely reversing cyclic stress and endurance strength A purely reversing or cyclic stress means when the stress alternates between equal positive and Pure cyclic stress negative peak stresses sinusoidally during each 300 cycle of operation, as shown. In this diagram the stress varies with time between +250 MPa 200 to -250MPa. This kind of cyclic stress is 100 developed in many rotating machine parts that 0 are carrying a constant bending load. -100 When a part is subjected cyclic stress, Stress (MPa) also known as range or reversing stress (Sr), it -200 has been observed that the failure of the part -300 occurs after a number of stress reversals (N) time even it the magnitude of Sr is below the material’s yield strength. Generally, higher the value of Sr, lesser N is needed for failure. No. of Cyclic stress stress (Sr) reversals for failure (N) psi 1000 81000 2000 75465 4000 70307 8000 65501 16000 61024 32000 56853 64000 52967 96000 50818 144000 48757 216000 46779 324000 44881 486000 43060 729000 41313 1000000 40000 For a typical material, the table and the graph above (S-N curve) show the relationship between the magnitudes Sr and the number of stress reversals (N) before failure of the part. For example, if the part were subjected to Sr= 81,000 psi, then it would fail after N=1000 stress reversals. If the same part is subjected to Sr = 61,024 psi, then it can survive up to N=16,000 reversals, and so on. Sengupta MET 301: Design for Cyclic Loading 1 of 7 It has been observed that for most of engineering materials, the rate of reduction of Sr becomes negligible near the vicinity of N = 106 and the slope of the S-N curve becomes more or less horizontal. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B. -

How Yield Strength and Fracture Toughness Considerations Can Influence Fatigue Design Procedures for Structural Steels
How Yield Strength and Fracture Toughness Considerations Can Influence Fatigue Design Procedures for Structural Steels The K,c /cfys ratio provides significant information relevant to fatigue design procedures, and use of NRL Ratio Analysis Diagrams permits determinations based on two relatively simple engineering tests BY T. W. CROOKER AND E. A. LANGE ABSTRACT. The failure of welded fatigue failures in welded structures probable mode of failure, the impor structures by fatigue can be viewed are primarily caused by crack propa tant factors affecting both fatigue and as a crack growth process operating gation. Numerous laboratory studies fracture, and fatigue design between fixed boundary conditions. The and service failure analyses have re procedures are discussed for each lower boundary condition assumes a peatedly shown that fatigue cracks group. rapidly initiated or pre-existing flaw, were initiated very early in the life of and the upper boundary condition the structure or propagated to failure is terminal failure. This paper outlines 1-6 Basic Aspects the modes of failure and methods of from pre-existing flaws. Recognition of this problem has The influence of yield strength and analysis for fatigue design for structural fracture toughness on the fatigue fail steels ranging in yield strength from 30 lead to the development of analytical to 300 ksi. It is shown that fatigue crack techniques for fatigue crack propaga ure process is largely a manifestation propagation characteristics of steels sel tion and the gathering of a sizeable of the role of plasticity in influencing dom vary significantly in response to amount of crack propagation data on the behavior of sharp cracks in struc broad changes in yield strength and frac a wide variety of structural materials. -

High Strength Steels
Automotive Worldwide Product Offer for US High Strength Steels Grade Availability ArcelorMittal offers the automotive industry a family of HI FORM (HF) high-strength steels with yield strength grades ranging from 250 to 550 MPa and tensile strength grades from 340 to 590 MPa. Yield Strength Grades Yield Strength Hot Rolled Cold Rolled Uncoated Uncoated EG HDGI HDGA 250 MPa - Yes Yes Yes - 300 MPa Yes Yes Yes - - 350 MPa Yes Yes Yes Yes Yes 400 MPa - Yes Yes Yes Yes 480, 500 MPa - Yes Yes - - 550 MPa - Yes Yes - - Tensile Strength Grades Tensile Strength Hot Rolled Cold Rolled Uncoated Uncoated EG HDGI HDGA 340 MPa - Yes Yes - Yes 370 MPa - Yes Yes - - 390 MPa - Yes Yes - Yes 440 MPa - Yes Yes - Yes 590 MPa Dev Yes - - Yes EG: Electrogalvanized HDGI: Hot-Dip-Galvanized HDGA: Hot-Dip-Galvannealed Yes: Products Commercially Available Note: Information contained in this document is subject to change. Please contact our sales team whenever you place an order to 1 ensure that your requirements are fully met. Please contact us if you have a specific requirement that is not included in the range of products and services covered by this document. Update: August 2009 Dev: Products are under development or in limited production. Please inquire about the status and availability of these products. Product Characteristics High strength steels are mainly used for structural applications and are hence also called structural steels. They can be produced with or without addition of small amount of microalloying elements. High strength low alloy steels, commonly referred to as HSLA steels, are produced using microalloying elements like Cb, Ti, Mo, V etc. -

An Investigation of the Low Temperature Creep Deformation Behavior of Titanium Grade 7 and Grade 5 Alloys—Progress Report with Preliminary Results
AN INVESTIGATION OF THE LOW TEMPERATURE CREEP DEFORMATION BEHAVIOR OF TITANIUM GRADE 7 AND GRADE 5 ALLOYS—PROGRESS REPORT WITH PRELIMINARY RESULTS Prepared for U.S. Nuclear Regulatory Commission Contract NRC–02–02–012 Prepared by S. Ankem,1 P.G. Oberson,2 R.V. Kazban,3 K.T. Chiang,3 L.F. Ibarra,3 and A.H. Chowdhury3 1Ankem Technology, Inc. Silver Spring, Maryland 2U.S. Nuclear Regulatory Commission Office of Nuclear Material Safety and Safeguards Division of High-Level Waste Repository Safety Washington, DC 3Center for Nuclear Waste Regulatory Analyses San Antonio, Texas August 2007 ABSTRACT The U.S. Nuclear Regulatory Commission (NRC) is interacting with U.S. Department of Energy (DOE) to gain insight into the potential geologic repository at Yucca Mountain, Nevada. In the design DOE is currently considering, the radioactive materials would be encapsulated in waste packages and emplaced in drifts (tunnels) below the surface. Subsequently, drip shields, primarily made of Titanium Grade 7 and Grade 24, would be placed over the waste packages to protect them from seepage and rockfall. If drift degradation occurs, drip shields would be subjected to impact loads due to the falling rock and static and dynamic (seismic) loads resulting from accumulated rockfall rubble. In the case when the accumulated rockfall rubble does not result in static loads high enough to produce immediate failure of the drip shield, the drip shield components would be subjected to permanent static loading, leading to stresses that may approach or exceed the yield stress of the drip shield materials. Titanium Grade 7 and Grade 5 (surrogate of Titanium Grade 24) were investigated for low temperature {from 25 to 250 °C [77 to 482 °F]} tensile and creep behavior. -

Very High Cycle Fatigue of Engineering Materials
Faculty of Technology and Science Materials Engineering Vitaliy Kazymyrovych Very high cycle fatigue of engineering materials (A literature review) Karlstad University Studies 2009:22 Vitaliy Kazymyrovych Very high cycle fatigue of engineering materials (A literature review) Karlstad University Studies 2009:22 Vitaliy Kazymyrovych. Very high cycle fatigue of engineering materials - A literature review Research Report Karlstad University Studies 2009:22 ISSN 1403-8099 ISBN 978-91-7063-246-4 © The Author Distribution: Faculty of Technology and Science Materials Engineering SE-651 88 Karlstad +46 54 700 10 00 www.kau.se Printed at: Universitetstryckeriet, Karlstad 2009 Very high cycle fatigue of engineering materials (A literature review) V.Kazymyrovych* Department of Materials Engineering, Karlstad University SE-651 88, Sweden * Email address: [email protected] Abstract This paper examines the development and present status of the Very High Cycle Fatigue (VHCF) phenomenon in engineering materials. The concept of ultrasonic fatigue testing is described in light of its historical appearance covering the main principles and equipment variations. The VHCF behaviour of the most important materials used for very long life applications is presented, with particular attention paid to steels. In section 3 the VHCF properties of titanium-, nickel-, aluminium- and magnesium alloys are described. Furthermore, the typical fatigue behaviour and mechanisms of pure metals are presented. Section 4 examines the VHCF properties of various types of steels e.g. low carbon steel, spring steel, stainless steel, bearing steel as well as tool steel. In addition to this, the main material defects that initiate VHCF failure are examined in this study. Furthermore, the different stages characteristic for fatigue crack development in VHCF are described in section 5 in terms of relative importance and sequence. -

From Yield to Fracture, Failure Initiation Captured by Molecular Simulation Laurent Brochard, Ignacio G
From yield to fracture, failure initiation captured by molecular simulation Laurent Brochard, Ignacio G. Tejada, Karam Sab To cite this version: Laurent Brochard, Ignacio G. Tejada, Karam Sab. From yield to fracture, failure initiation captured by molecular simulation. Journal of the Mechanics and Physics of Solids, Elsevier, 2016, 95, pp.632 - 646. 10.1016/j.jmps.2016.05.005. hal-01686201 HAL Id: hal-01686201 https://hal.archives-ouvertes.fr/hal-01686201 Submitted on 20 Jun 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. From Yield to Fracture, Failure Initiation Captured by Molecular Simulation Laurent Brocharda,∗, Ignacio G. Tejadab, Karam Saba aUniversit´eParis-Est, Laboratoire Navier (UMR 8205), CNRS, ENPC, IFSTTAR 6 & 8 avenue Blaise Pascal, 77455 Marne-la-Vall´ee,France. bUniversit´eGrenoble Alpes, 3SR, CNRS Domaine Universitaire BP53, 38041 Grenoble Cedex 9, France Abstract While failure of cracked bodies with strong stress concentrations is described by an energy criterion (fracture mechanics), failure of flawless bodies with uniform stresses is captured by a criterion on stress (yielding). In-between those two cases, the problem of failure initiation from flaws that moderately concentrate stresses is debated. -

Hardness - Yield Strength Relation of Al-Mg-Si Alloys with Enhanced Strength and Conductivity V.D
IOP Conference Series: Materials Science and Engineering PAPER • OPEN ACCESS Related content - Precipitates studies in ultrafine-grained Al Hardness - Yield Strength Relation of Al-Mg-Si alloys with enhanced strength and conductivity V.D. Sitdikov, M.Yu. Murashkin and R.Z. Alloys Valiev - The Contrast of Electron Microscope To cite this article: Aluru Praveen Sekhar et al 2018 IOP Conf. Ser.: Mater. Sci. Eng. 338 012011 Images of Thick Crystalline Object. Experiment with an Al-Mg-Si Alloy at 50- 100 kV. Toshio Sahashi - Microstructure features and mechanical View the article online for updates and enhancements. properties of a UFG Al-Mg-Si alloy produced via SPD E Bobruk, I Sabirov, V Kazykhanov et al. This content was downloaded from IP address 170.106.33.14 on 24/09/2021 at 15:30 NCPCM 2017 IOP Publishing IOP Conf. Series: Materials Science and Engineering1234567890 338 (2018)‘’“” 012011 doi:10.1088/1757-899X/338/1/012011 Hardness - Yield Strength Relation of Al-Mg-Si Alloys Aluru Praveen Sekhar*, Supriya Nandy, Kalyan Kumar Ray and Debdulal Das Department of Metallurgy and Materials Engineering, Indian Institute of Engineering Science and Technology, Shibpur, Howrah - 711103, West Bengal, India *Corresponding author E-mail address: [email protected] Abstract: Assessing the mechanical properties of materials through indentation hardness test is an attractive method, rather than obtaining the properties through destructive approach like tensile testing. The present work emphasizes on the relation between hardness and yield strength of Al-Mg-Si alloys considering Tabor type equations. Al-0.5Mg-0.4Si alloy has been artificially aged at various temperatures (100 to 250 oC) for different time durations (0.083 to 1000 h) and the ageing response has been assessed by measuring the Vickers hardness and yield strength.