The Peppered Moth: Decline of a Darwinian Disciple Michael E.N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Database Integrating Genomic Indel Polymorphisms That Distinguish Mouse Strains Keiko Akagi1,2, Robert M
D600–D606 Nucleic Acids Research, 2010, Vol. 38, Database issue Published online 20 November 2009 doi:10.1093/nar/gkp1046 MouseIndelDB: a database integrating genomic indel polymorphisms that distinguish mouse strains Keiko Akagi1,2, Robert M. Stephens3, Jingfeng Li2,4, Evgenji Evdokimov5, Michael R. Kuehn5, Natalia Volfovsky3 and David E. Symer2,4,6,7,8,9,* 1Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702, 2Department of Molecular Virology, Immunology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, 3Advanced Biomedical Computing Center, Information Systems Program, SAIC-Frederick, Inc., NCI-Frederick, Frederick, MD 21702, 4Basic Research Laboratory, 5Laboratory of Protein Dynamics and Signaling, 6Laboratory of Biochemistry and Molecular Biology, Center for Cancer Research, National Cancer Institute, Frederick, MD 21702, 7Human Cancer Genetics Program, 8Departments of Internal Medicine and 9Biomedical Informatics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210, USA Received August 5, 2009; Revised October 23, 2009; Accepted October 27, 2009 ABSTRACT wide-ranging functional differences. This extensive phenotypic variation has helped to make the mouse a MouseIndelDB is an integrated database resource premier model organism, mimicking many aspects of containing thousands of previously unreported human diversity and diseases. Understanding the mouse genomic indel (insertion and deletion) genomic differences that -

Pathway in Protective Immunity Jeremy Manry, Guillaume Laval, Etienne Patin, Simona Fornarino, Christiane Bouchier, Magali Tichit, Luis Barreiro, Lluis Quintana-Murci
Evolutionary genetics evidence of an essential, non-redundant role of the IFN-γ pathway in protective immunity Jeremy Manry, Guillaume Laval, Etienne Patin, Simona Fornarino, Christiane Bouchier, Magali Tichit, Luis Barreiro, Lluis Quintana-Murci To cite this version: Jeremy Manry, Guillaume Laval, Etienne Patin, Simona Fornarino, Christiane Bouchier, et al.. Evo- lutionary genetics evidence of an essential, non-redundant role of the IFN-γ pathway in protective immunity. Human Mutation, Wiley, 2011, 32 (6), pp.633-42. 10.1002/humu.21484. hal-00627541 HAL Id: hal-00627541 https://hal.archives-ouvertes.fr/hal-00627541 Submitted on 29 Sep 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Human Mutation Evolutionary genetics evidence of an essential, non- redundant role of the IFN-γ pathway in protective immunity For Peer Review Journal: Human Mutation Manuscript ID: humu-2010-0462.R1 Wiley - Manuscript type: Research Article Date Submitted by the 23-Jan-2011 Author: Complete List of Authors: Manry, Jeremy; Institut Pasteur Laval, Guillaume; Institut Pasteur Patin, Etienne; Institut Pasteur Fornarino, Simona; Institut Pasteur Bouchier, Christiane; Institut Pasteur Tichit, Magali; Institut Pasteur Barreiro, Luis; University of Chicago Quintana-Murci, Lluis; Institut Pasteur, EEMI IFNG, IFNGR1, IFNGR2, natural selection, polymorphism, Key Words: population genetics John Wiley & Sons, Inc. -
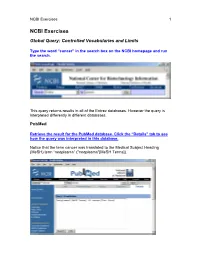
NCBI Exercises 1
NCBI Exercises 1 NCBI Exercises Global Query: Controlled Vocabularies and Limits Type the word “cancer” in the search box on the NCBI homepage and run the search. This query returns results in all of the Entrez databases. However the query is interpreted differently in different databases. PubMed Retrieve the result for the PubMed database. Click the “Details” tab to see how the query was interpreted in this database. Notice that the term cancer was translated to the Medical Subject Heading (MeSH) term “neoplasms” ("neoplasms"[MeSH Terms]). NCBI Exercises 2 MeSH is a controlled vocabulary that is used to index all articles in PubMed. In the details box, edit the query to remove the portion that searched for cancer as a text word and run the search. Notice that the number of articles retrieved has changed. These will be a more relevant set of results. You can force the PubMed engine to only search the MeSH vocabulary or specify any other indexed field through the “Limits” tab. Use the Web browser’s back button to return to the Global query page and retrieve the PubMed results again. Now click on the “Limits” tab. Select “MeSH terms” from the first drop-down menu, the one headed by “All Fields”. Now run the search with the limit in place and check the “Details” tab to verify that only the MeSH term translation was used. Nucleotide Use the Web browser’s back button to return to the Global query page. Retrieve the results for the nucleotide database. Click the “Details” tab to see how the query was interpreted for this molecular database. -

Why Why Darwin Matters Matters Why Darwin Matters: the Case Against Intelligent Design, by Michael Shermer
Evo Edu Outreach DOI 10.1007/s12052-008-0109-9 BOOK REVIEW Why Why Darwin Matters Matters Why Darwin Matters: The Case Against Intelligent Design, by Michael Shermer. New York: Henry Holt, 2006. Pp. xxii + 199. S/b $14.00 Tania Lombrozo # The Author(s) 2008. This article is published with open access at Springerlink.com The first decade of the twenty-first century will be a curious a brief but lucid overview of key evolutionary ideas. He chapter in the future history of evolutionary thought. In emphasizes that the source of resistance to evolution is rarely 2005, resistance to evolution manifested in the highly the scientific details but rather the perceived consequences of publicized trial of Dover, Pennsylvania over teaching evolution: atheism, ethical nihilism, and a lack of meaning. Intelligent Design in public schools. Only four years later, What people care about “is whether teaching evolution will in 2009, universities, museums, and individuals across the make their kids reject God, allow criminals and sinners to globe celebrate the 150th anniversary of the publication of blame their genes for their actions, and generally cause society Darwin’s Origin of Species. Released in the interlude, to fall apart” (p. 25). But according to Shermer, an even Michael Shermer’s Why Darwin Matters: The Case Against greater threat to the theory of evolution is misunderstanding. Intelligent Design takes on the challenge these landmark A large proportion of the public not only misunderstands dates represent: how a thoroughly vetted and accepted evolutionary theory but also aspects of science and the scientific theory can be the source of so much cultural scientific process—for example, that calling evolution a conflict. -

Ten Misunderstandings About Evolution a Very Brief Guide for the Curious and the Confused by Dr
Ten Misunderstandings About Evolution A Very Brief Guide for the Curious and the Confused By Dr. Mike Webster, Dept. of Neurobiology and Behavior, Cornell Lab of Ornithology, Cornell University ([email protected]); February 2010 The current debate over evolution and “intelligent design” (ID) is being driven by a relatively small group of individuals who object to the theory of evolution for religious reasons. The debate is fueled, though, by misunderstandings on the part of the American public about what evolutionary biology is and what it says. These misunderstandings are exploited by proponents of ID, intentionally or not, and are often echoed in the media. In this booklet I briefly outline and explain 10 of the most common (and serious) misunderstandings. It is impossible to treat each point thoroughly in this limited space; I encourage you to read further on these topics and also by visiting the websites given on the resource sheet. In addition, I am happy to send a somewhat expanded version of this booklet to anybody who is interested – just send me an email to ask for one! What are the misunderstandings? 1. Evolution is progressive improvement of species Evolution, particularly human evolution, is often pictured in textbooks as a string of organisms marching in single file from “simple” organisms (usually a single celled organism or a monkey) on one side of the page and advancing to “complex” organisms on the opposite side of the page (almost invariably a human being). We have all seen this enduring image and likely have some version of it burned into our brains. -

Intelligent Design Creationism and the Constitution
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Washington University St. Louis: Open Scholarship Washington University Law Review Volume 83 Issue 1 2005 Is It Science Yet?: Intelligent Design Creationism and the Constitution Matthew J. Brauer Princeton University Barbara Forrest Southeastern Louisiana University Steven G. Gey Florida State University Follow this and additional works at: https://openscholarship.wustl.edu/law_lawreview Part of the Constitutional Law Commons, Education Law Commons, First Amendment Commons, Religion Law Commons, and the Science and Technology Law Commons Recommended Citation Matthew J. Brauer, Barbara Forrest, and Steven G. Gey, Is It Science Yet?: Intelligent Design Creationism and the Constitution, 83 WASH. U. L. Q. 1 (2005). Available at: https://openscholarship.wustl.edu/law_lawreview/vol83/iss1/1 This Article is brought to you for free and open access by the Law School at Washington University Open Scholarship. It has been accepted for inclusion in Washington University Law Review by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. Washington University Law Quarterly VOLUME 83 NUMBER 1 2005 IS IT SCIENCE YET?: INTELLIGENT DESIGN CREATIONISM AND THE CONSTITUTION MATTHEW J. BRAUER BARBARA FORREST STEVEN G. GEY* TABLE OF CONTENTS ABSTRACT ................................................................................................... 3 INTRODUCTION.................................................................................................. -

"Critical Analysis of Evolution"; Innovative Lesson Plan Or
THE OHIO DEPARTMENT OF EDUCATION L10H23 “CRITICAL ANALYSIS OF EVOLUTION”; INNOVATIVE LESSON PLAN OR STEALTHY ADVOCACY TOOL? Robert Day, The Ohio State University. Presented at the National Association of Researchers of Science Teaching (NARST) annual conference, San Francisco, CA. April 2006. Abstract: This paper will discuss the ongoing controversy surrounding a particular Ohio Department of Education tenth grade lesson plan titled “Critical Analysis of Evolution” (Ohio Department of Education identification L10H23). The lesson professes to encourage students to “critically examine” evidences for and against evolution and invites them to discuss definitions of some common evolutionary terms and concepts. Proponents insist that this lesson is a thought-provoking exercise in critical thinking and scientific objectively. Critics claim that the lesson is at best, unscientific and at worst, a thinly-veiled attempt to introduce creationist ideas into the classroom in accordance with the so-called “wedge” strategy of certain pro-creationist organizations. A complicating factor is that this lesson plan has been used as the subject of graduate level research on the effect of teaching “the evolution controversy” to Ohio students, and subsequently, this research has been used to support similar initiatives in state hearings outside of Ohio. We will present the findings from a series of surveys conducted with life-science high school teachers, college faculty, and graduate students intended to establish whether or not practicing scientists and science educators agree with the Ohio Board of Education’s assessment that “there is no ID [intelligent design] there”. We will look for trends in the opinions of different sub-populations, identify key differences of opinions between participants and Ohio Board of Education members and suggest possible reasons for any apparent conflicts of opinion. -

Why Much of What We Teach About Evolution Is Wrong/By Jonathan Wells
ON SCIENCE OR MYTH? Whymuch of what we teach about evolution is wrong Icons ofEvolution About the Author Jonathan Wells is no stranger to controversy. After spending two years in the U.S. Ar my from 1964 to 1966, he entered the University of California at Berkeley to become a science teacher. When the Army called him back from reser ve status in 1968, he chose to go to prison rather than continue to serve during the Vietnam War. He subsequently earned a Ph.D. in religious studies at Yale University, where he wrote a book about the nineteenth century Darwinian controversies. In 1989 he returned to Berkeley to earn a second Ph.D., this time in molecular and cell biology. He is now a senior fellow at Discovery Institute's Center for the Renewal of Science and Culture (www.discovery.org/ crsc) in Seattle, where he lives with his wife, two children, and mother. He still hopes to become a science teacher. Icons ofEvolution Science or Myth? Why Much oJWhat We TeachAbout Evolution Is Wrong JONATHAN WELLS ILLUSTRATED BY JODY F. SJOGREN IIIIDIDIREGNERY 11MPUBLISHING, INC. An EaglePublishing Company • Washington, IX Copyright © 2000 by Jonathan Wells All rights reserved. No part of this publication may be reproduced or trans mitted in any form or by any means electronic or mechanical, including pho tocopy, recording, or any information storage and retrieval system now known or to be invented, without permission in writing from the publisher, except by a reviewer who wishes to quote brief passages in connection with a review written for inclusion in a magazine, newspaper, or broadcast. -

Peppered Moths and the Industrial Revolution: Barking up the Wrong Tree? by Avril M
NATIONAL CENTER FOR CASE STUDY TEACHING IN SCIENCE Peppered Moths and the Industrial Revolution: Barking up the Wrong Tree? by Avril M. Harder1, Janna R. Willoughby1,2, Jaqueline M. Doyle3 Part I – Hypotheses and Predictions The story of how Britain’s Industrial Revolution led to changes in peppered moth coloration is a classic, textbook ex- ample of evolution in action. You might even already be familiar with the plot and the findings of the main researcher of the phenomenon, H.B.D. Kettlewell. Although the peppered moth story has been told and re-told in thousands of biology classrooms, most narratives leave out an important bit of history: after Kettlewell published his results that described changes in morph population frequencies over time, many of his peers questioned the validity of his data. Your first task in this case study will be to put yourself in the place of one of Kettlewell’s contemporaries, evaluate his experimental methods, and decide whether his data support his assertions. Background Biston betularia is a medium-sized, nocturnal moth. Al- though the peppered moth is found across the world, some of the first research on this species was conducted in Great Britain. Before the Industrial Revolution, tree trunks in unpolluted areas were covered with lichens, and peppered moths with light colored wings were well camouflaged against this background (Figure 1). In this environment, light morphs vastly outnumbered dark morphs. During the Industrial Revolution, increasing air pollution led to the formation of acid rain and inhospitable conditions for the lichens. The death of the lichens combined with soot deposition on tree trunks led to an important environmen- tal shift for the moths. -

Garden Moth Scheme Report 2017
Garden Moth Scheme Report 2017 Heather Young – April 2018 1 GMS Report 2017 CONTENTS PAGE Introduction 2 Top 30 Species 2017 3 Scientific Publications 4 Abundant and Widespread Species 8 Common or Garden Moths 11 Winter GMS 2017-18 15 Coordination Changes 16 GMS Annual Conference 16 GMS Sponsors 17 Links & Acknowledgements 18 Cover photograph: Peppered Moth (H. Young) Introduction The Garden Moth Scheme (GMS) welcomes participants from all parts of the United Kingdom and Ireland, and in 2017 received 360 completed recording forms, an increase of over 5% on 2016 (341). We have consistently received records from over 300 sites across the UK and Ireland since 2010, and now have almost 1 ½ million records in the GMS database. Several scientific papers using the GMS data have now been published in peer- reviewed journals, and these are listed in this report, with the relevant abstracts, to illustrate how the GMS records are used for research. The GMS is divided into 12 regions, monitoring 233 species of moth in every part of the UK and Ireland (the ‘Core Species’), along with a variable number of ‘Regional Species’. A selection of core species whose name suggests they should be found commonly, or in our gardens, is highlighted in this report. There is a round-up of the 2017-18 Winter Garden Moth Scheme, which attracted a surprisingly high number of recorders (102) despite the poor weather, a summary of the changes taking place in the GMS coordination team for 2018, and a short report on the 2018 Annual Conference, but we begin as usual with the Top 30 for GMS 2017. -

How Is the Peppered Moth Evidence of Natural Selection Over a Short Period of Time? Lab # ______
Name: _______________________________ Date: ________________ How is the peppered moth evidence of natural selection over a short period of time? Lab # ______ Step 1- Background information: Charles Darwin collected a large amount of facts to create the theory of evolution by natural selection. Darwin described natural selection as the “process by which the organisms that are best adapted to a specific environment survive and produce more offspring than organisms that are not as well adapted”. One of his difficulties in proving the theory, however, was the lack of an example of evolution over a short period of time, which could be observed during ones lifetime. Although Darwin was unaware of it, remarkable examples of evolution, which might have helped to persuade people of his theory, were in the countryside of his native England. One such example is the evolution of the peppered moth, Biston betularia. 1) Describe Darwin’s theory of natural selection in your own words. ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ 2) What was one difficulty Darwin had supporting his theory, even though he had tremendous (large) amounts of evidence? ___________________________________________________________________________________________________________ ___________________________________________________________________________________________________________ 3) Why was his theory so -

(12) Patent Application Publication (10) Pub. No.: US 2008/0085284 A1 Patell Et Al
US 20080O85284A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0085284 A1 Patell et al. (43) Pub. Date: Apr. 10, 2008 (54) CONSTRUCTION OF A COMPARATIVE (52) U.S. Cl. ............................. 424/184.1; 435/6: 514/44; DATABASE AND IDENTIFICATION OF 536/23.7:536/24.33: 707/102 VIRULENCE FACTORS COMPARISON OF POLYMORPHC REGIONS IN CLINICAL (57) ABSTRACT SOLATES OF INFECTIOUS ORGANISMS The present invention is directed to novel nucleotide (76) Inventors: Villoo Morawala Patell, Bangalore sequences to be used for diagnosis, identification of the (IN); K.R. Rajyashri, Bangalore (IN); strain, typing of the strain and giving orientation to its Marc Rodrigue, Marcy (FR); Guy potential degree of virulence, infectivity and/or latency for Vernet, Marcy (FR) all infectious diseases more particularly tuberculosis. The present invention also includes method for the identification Correspondence Address: and selection of polymorphisms associated with the viru SALWANCHIK LLOYD & SALWANCHK lence and/or infectivity in infectious diseases more particu A PROFESSIONAL ASSOCATION larly in tuberculosis by a comparative genomic analysis of PO BOX 142950 the sequences of different clinical isolates/strains of infec GAINESVILLE, FL 32614-2950 (US) tious organisms. The regions of polymorphisms, can also act (21) Appl. No.: 11/632,108 as potential drug targets and vaccine targets. More particu larly, the invention also relates to identifying virulence (22) Filed: Apr. 9, 2007 factors of M. tuberculosis strains and other infectious organ isms to be included in a diagnostic DNA chip allowing Publication Classification identification of the strain, typing of the strain and finally (51) Int. Cl.