Impact of Quality of Ground Water Irrigation on Soil Health in Bilara
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rajasthan List.Pdf
Interview List for Selection of Appointment of Notaries in the State of Rajasthan Date Of Area Of S.No Name Category Father's Name Address Enrol. No. & Date App'n Practice Village Lodipura Post Kamal Kumar Sawai Madho Lal R/2917/2003 1 Obc 01.05.18 Khatupura ,Sawai Gurjar Madhopur Gurjar Dt.28.12.03 Madhopur,Rajasthan Village Sukhwas Post Allapur Chhotu Lal Sawai Laddu Lal R/1600/2004 2 Obc 01.05.18 Tehsil Khandar,Sawai Gurjar Madhopur Gurjar Dt.02.10.04 Madhopur,Rajasthan Sindhu Farm Villahe Bilwadi Ram Karan R/910/2007 3 Obc 01.05.18 Shahpura Suraj Mal Tehsil Sindhu Dt.22.04.07 Viratnagar,Jaipur,Rajasthan Opposite 5-Kha H.B.C. Sanjay Nagar Bhatta Basti R/1404/2004 4 Abdul Kayam Gen 02.05.18 Jaipur Bafati Khan Shastri Dt.02.10.04 Nagar,Jaipur,Rajasthan Jajoria Bhawan Village- Parveen Kumar Ram Gopal Keshopura Post- Vaishali R/857/2008 5 Sc 04.05.18 Jaipur Jajoria Jajoria Nagar Ajmer Dt.28.06.08 Road,Jaipur,Rajasthan Kailash Vakil Colony Court Road Devendra R/3850/2007 6 Obc 08.05.18 Mandalgarh Chandra Mandalgarh,Bhilwara,Rajast Kumar Tamboli Dt.16.12.07 Tamboli han Bhagwan Sahya Ward No 17 Viratnagar R/153/1996 7 Mamraj Saini Obc 03.05.18 Viratnagar Saini ,Jaipur,Rajasthan Dt.09.03.96 156 Luharo Ka Mohalla R/100/1997 8 Anwar Ahmed Gen 04.05.18 Jaipur Bashir Ahmed Sambhar Dt.31.01.97 Lake,Jaipur,Rajasthan B-1048-49 Sanjay Nagar Mohammad Near 17 No Bus Stand Bhatta R/1812/2005 9 Obc 04.05.18 Jaipur Abrar Hussain Salim Basti Shastri Dt.01.10.05 Nagar,Jaipur,Rajasthan Vill Bislan Post Suratpura R/651/2008 10 Vijay Singh Obc 04.05.18 Rajgarh Dayanand Teh Dt.05.04.08 Rajgarh,Churu,Rajasthan Late Devki Plot No-411 Tara Nagar-A R/41/2002 11 Rajesh Sharma Gen 05.05.18 Jaipur Nandan Jhotwara,Jaipur,Rajasthan Dt.12.01.02 Sharma Opp Bus Stand Near Hanuman Ji Temple Ramanand Hanumangar Rameshwar Lal R/29/2002 12 Gen 05.05.18 Hanumangarh Sharma h Sharma Dt.17.01.02 Town,Hanumangarh,Rajasth an Ward No 23 New Abadi Street No 17 Fatehgarh Hanumangar Gangabishan R/3511/2010 13 Om Prakash Obc 07.05.18 Moad Hanumangarh h Bishnoi Dt.14.08.10 Town,Hanumangarh,Rajasth an P.No. -

Udaipur & Jodhpur
ICICI Rural Self Employment Training Institute # ANNUAL REPORT 2019-20 Udaipur & Jodhpur Learn today About Us Vision For over six decades, the ICICI Group has contributed To drive inclusive growth in the mandated regions by for a significantly towards India's economic growth and contributing to the key enablers for widespread development. Promoting inclusive growth has been a participation in economic opportunities. key priority for the Group. We have not only made meaningful differences in the lives of our customers Mission through our suite of products and services but have better also been consistently supporting development To drive inclusive growth in mandated regions through initiatives and community outreach efforts. ICICI focused initiatives in various identified areas, including Foundation, since its inception in 2008, has been the skill development, enabling sustainable livelihood, catalyst for inclusive growth, has been supporting financial inclusion and entrepreneurship development. and enhancing livelihood projects across India tomorrow through its three verticals – ICICI Academy for Skills, ICICI Rural Livelihood and ICICI RSETI. ICICI Foundation oversees the activities of the two ICICI RSETIs (Rural Self Employment Training Institutes) at Udaipur and Jodhpur - Rajasthan. Under the aegis of Ministry of Rural Development – Government of India (MoRD) since the year 2011, ICICI Bank supports the functioning of the two RSETIs in Rajasthan with National Centre of Excellence (NACER) being the nodal monitoring agency. ICICI RSETIs works on creating sustainable livelihood opportunities for marginalised and the lesser privileged by imparting vocational training programs, facilitating credit and market linkages and handholding support for two years. ICICI RSETI has evolved as a core establishment for providing sustainable livelihood in the region. -

District Boundary Assembly Constituency
NEW ASSEMBLY CONSTITUENCIES (Based on Rajasthan Gazette Notification SADULSHAHAR-1 Dated 25th January, 2006) GANGANAGAR-2 SANGARIA-7 KARANPUR-3 RAJASTHAN PILIBANGA (SC)-9 HANUMANGARH- 8 GANGANAGAR RAISINGHNAGAR (SC)-5 SURATGARH-4 HANUMANGARH BHADRA-11 NOHAR-10 ® ANUPGARH (SC)-6 TARANAGAR-20 LUNKARANSAR-16 SADULPUR-19 KHAJUWALA SARDARSHAHAR-21 (SC)-12 CHURU PILANI CHURU-22 (SC)-25 BIKANER MANDAWA-28 SURAJGARH-26 JHUNJHUNUN-27 TIJARA-59 JHUNJHUNUN DUNGARGARH-17 BIKANER FATEHPUR-32 WEST-13 KHETRI-31 BEHROR- BIKANER RATANGARH-23 EAST-14 NAWALGARH- 62 MUNDAWAR- 61 29 KISHANGARH KOLAYAT-15 UDAIPURWATI- BAS-60 LACHHMANGARH-33 30 NEEM KA THANA-38 KAMAN-70 SUJANGARH KOTPUTLI-40 (SC)-24 SIKAR-35 BANSUR-63 KHANDELA-37 ALWAR LADNUN- URBAN-66 RAMGARH-67 NOKHA-18 106 DHOD (SC)-34 SIKAR SRIMADHOPUR-39 ALWAR NAGAR-71 ALWAR VIRATNAGAR-41 RURAL (SC)-65 DEEG - DANTA KUMHER-72 RAMGARH-36 KATHUMAR DEEDWANA-107 SHAHPURA-42 PHALODI-122 (SC)-69 JAYAL RAJGARH - (SC)-108 BHARATPUR- NAGAUR- CHOMU-43 THANAGAZI-64 LAXMANGARH (ST)-68 73 109 NADBAI-74 NAWAN- JAISALMER- 115 JAMWA BHARATPUR 132 AMBER-47 MAHUWA-86 NAGAUR MAKRANA- RAMGARH BANDIKUI-85 WEIR 113 (ST)-48 (SC)-75 JAISALMER KHINVSAR-110 JHOTWARA-46 JAIPUR DAUSA- LOHAWAT-123 BAYANA POKARAN- ADARSH 88 NAGAR-53 DAUSA (SC)-76 133 PHULERA-44 SIKRAI RAJAKHERA-80 DEGANA- (SC)-87 HINDAUN 112 PARBATSAR- BASSI (SC)-82 BAGRU 114 (ST)-57 PUSHKAR- (SC)-56 TODABHIM DHOLPUR- JODHPUR 99 (ST)-81 DHAULPUR 79 OSIAN- DUDU BARI-78 125 (SC)-45 CHAKSU KARAULI-83 MERTA (SC)-58 LALSOT BAMANWAS BASERI BHOPALGARH (SC)-111 (ST)-89 (SC)-126 -

Final Population Figures, Series-18, Rajasthan
PAPER 1 OF 1982 CENSUS OF INDIA 1981 SERIES 18 RAJASTHAN fINAL POPULATION FIGU~ES (TOTAL POPULATION, SCHEDULED CASTE POPULATION AND .sCHEDULED TRIBE POPULATION) I. C. SRIVASTAVA ·1)f the Indian Administrative Service Director of Census Operations Rajasthan INTRODUCfION The final figures of total population, scheduled caste and scheduled tribe population of Rajasthan Stat~ are now ready for release at State/District/Town and Tehsil levels. This Primary Census Abs tract, as it is called, as against the provisional figures contained in our three publications viz. Paper I, fFacts & Figures' and Supplement to Paper-I has been prepared through manual tabulation by over 1400 census officials including Tabulators, Checkers and Supervisors whose constant and sustained efforts spread over twelve months enabled the Directorate to complete the work as per the schedule prescribed at the national level. As it will take a few months more to publish the final population figures at the viJ1age as well as ward levels in towns in the form of District Census Handbooks, it is hoped, this paper will meet the most essential and immediate demands of various Government departments, autonomous bodies, Cor porations, Universities and rtsearch institutions in relation to salient popUlation statistics of the State. In respect of 11 cities with One lac or more population, it has also been possible to present ~the data by municipal wards as shown in Annexure. With compliments from Director of Census Operations, Rajasthan CONTENTS INTRODUCTION (iii) Total Population, Scheduled Caste and Scheduled Tribt' Population by Districts, 1981 Total Schedu1ed Caste and Scheduled Tribe Population. ( vi) 1. Ganganagar District 1 2. -

Annexure-EIA/EMP
Prepared by M/s Udaipur Min-Tech Pvt. Ltd. STUDY PERIOD-DEC, 2013 TO FEB , 2014 NON-FOREST LAND ENVIRONMENTAL IMPACT ASSESSMENT ENVIRONMENTAL MANAGEMENT PLAN FOR RIVER BED MINING PROJECT OF MINOR MINERAL ORDINARY SAND(BAJRI) IN Tehsil: Bilara, District: Jodhpur, Rajasthan NAME OF RIVER – LUNI & MITRI RIVER AND ITS CONNECTED FLOW NALLAHS Purpose – Fresh Grant, Proposed Production – 40,00,000 MTPA APPLIED LEASE AREA– 2439Hect. PROJECT COST – 2.5 CRORE. CATEGORY- ΄A΄ APPLICANT EIA CONSULTANT Sh. Himmat Singh Sekhawat M/S UDAIPUR MIN-TECH PVT. LTD 98, Rooprajat Township 206-APEKSHA COMPLEX Phase-II,Pal Road, SECTOR NO.-11 Jodhpur, Rajasthan HIRAN MAGARI, UDAIPUR-313002 (RAJ.) Mobs. 09799955444 PH- 91-294-2489672 (OFF.) E mail:- yagyapratap@gmailcom E mail :- [email protected] ACCREDITED BY NABET (S.NO. 159) CATEGORY “A” FOR SECTOR 1 & ‘B’ CATEGORY FOR SECTOR 22 & 38 M/s Himmat Singh Shekhawat River Bed Mining 1 Prepared by M/s Udaipur Min-Tech Pvt. Ltd. CONTENTS CHAPTER PARTICULAR PAGE NO. NO. 0 ACCREDITATION CERTIFICATE OF NABET 3 0 COMPLIANCE TO TERM OF REFERENCE 32 (TOR) I INTRODUCTION 34-41 II PROJECT DESCRIPTION 42-54 III DESCRIPTION OF THE ENVIRONMENT 55-157 IV ANTICIPATED ENVIRONMENTAL IMPACTS 158-172 & MITIGATION MEASURES V ANALYSIS OF ALTERNATIVES 173-174 VI ENVIRONMENTAL MONITORING 175-178 PROGRAMME VII ADDITIONAL STUDIES 179-192 VIII PROJECT BENEFITS 193-195 IX ENVIRONMENTAL MANAGEMENT PLAN 196-205 X SUMMARY &CONCLUSION 206-209 XI DISCLOSURE OF CONSULTANTS ENGAGED 210-212 M/s Himmat Singh Shekhawat River Bed Mining 2 Prepared by M/s Udaipur Min-Tech Pvt. Ltd. -

List of Rajasthan Pradesh Congress Seva Dal Office Bearers-2017
List of Rajasthan Pradesh Congress Seva Dal Office bearers-2017 Chief Organiser 1 Shri Rakesh Pareek Shri Rakesh Pareek Chief Organiser Chief Organiser Rajasthan Pradesh Congress Seva Dal Rajasthan Pradesh Congress Seva Dal B-613 Sawai Jaisingh Highway, Vill/PO-Sarvad Ganeshganj Banipark Ajmer Jaipur Rajasthan Rajasthan Tel-09414419400 Mahila Organiser 1 Smt. Kalpana Bhatnagar Mahila Organiser Rajasthan Pradesh Congress Seva Dal 46, Navrang Nagar Beawar, Dist- Ajmer Rajasthan Tel: 09001864018 Additional Chief OrganisersP 1 Shri Hajari Lal Nagar 2 Shri Ram Kishan Sharma Additional Chief Organiser Additional Chief Organiser Rajasthan Pradesh Congress Seva Dal Rajasthan Pradesh Congress Seva Dal C 4/272 Vidyadhar Nagar Ghanshyam Ji Ka Mandir Jaipur (Rajasthan) Gangapol Bahar, Badanpura Tel:- 09214046342, 09414446342 Jaipur 09829783637 Rajasthan Tel:- 09314504631 3 Shri Hulas Chand Bhutara 4 Shri Manjoor Ahmed Additional Chief Organiser Additional Chief Organiser Rajasthan Pradesh Congress Seva Dal Rajasthan Pradesh Congress Seva Dal C-53, Panchshel Colony 4354, Mohalla Kayamkhani Purani Chungi Topkhano Ka Rasta Ajmer Road Chandpol Bazar Jaipur--302019 Jaipur Rajasthan Rajasthan Tel: 01531-220642, 09414147159 Tel: 09314603489, 08890473767 09079004827 5 Shri Bhawani Mal Ajmera 6 Shri Ram Bharosi Saini Additional Chief Organiser Additional Chief Organiser Rajasthan Pradesh Congress Seva Dal Rajasthan Pradesh Congress Seva Dal Rahul Electricals, V/Post- Chantali Ganesh Shopping Teh- Wair Complex, Opp.R No-2, Dist- Bharatpur VKI Chonu Rd. Rajasthan -

Assessment of Ground Water Quality of Rajasthan with Special Reference to Bilara (Jodhpur) Region
www.ijcrt.org © 2018 IJCRT | Volume 6, Issue 2 April 2018 | ISSN: 2320-2882 ASSESSMENT OF GROUND WATER QUALITY OF RAJASTHAN WITH SPECIAL REFERENCE TO BILARA (JODHPUR) REGION 1Mamta Patel, 2Sanwal Ram, 3Vimla Chowdhary 1Research Scholar, 2Research Scholar, 3Professor & Research Supervisor, Department of Chemistry, Jai Narain Vyas University, Jodhpur, Rajasthan, India ABSTRACT: A well planned and systematic study was carried out to make assess of the water quality of Bilara region (Jodhpur district) of Rajasthan. The focus of the study was to determine the physico–chemical parameters of ground water samples collected from the populated regions of Bilara, where the same water is used as potable water. A comprehensive physico– chemical analysis of some parameters such as pH, conductivity, total dissolved solids (TDS), total hardness (TH), Calcium, Magnesium, Nitrate, Chloride, Fluoride, etc was done. The analysis reveal that some of the samples contained chemical constituent beyond permissible limits prescribed by Bureau of Indian Standards (B.I.S), which may result to various hazardous effects to the health of the people residing in those areas. In this paper, regional ground water contamination will broadly refer to the types of ground water contamination that can be observed at the regional scale. Based on the results of this study, it is recommended that any ground water source which comes under this study area should be tested before use for its portability and other domestic or industrial uses. Key words: Ground water quality, Water Quality Index, Physico-chemical parameters, Bilara (Jodhpur) region. INTRODUCTION:- When asked about the most essential thing for survival then no doubt the answer is: Water! It is essential for the survival of all living forms of the earth. -

Palaeoredox Link with the Late Neoproterozoic–Early Cambrian Bilara Carbonate Deposition, Marwar Supergroup, India
Carbonates and Evaporites (2020) 35:38 https://doi.org/10.1007/s13146-020-00574-9 ORIGINAL ARTICLE Palaeoredox link with the late Neoproterozoic–early Cambrian Bilara carbonate deposition, Marwar Supergroup, India A. H. Ansari1 · S. K. Pandey1 · Kamlesh Kumar1 · Shailesh Agrawal1 · Shamim Ahmad1 · Mayank Shekhar1 Accepted: 27 February 2020 © Springer-Verlag GmbH Germany, part of Springer Nature 2020 Abstract This study performed a stable isotope and redox-sensitive trace-element investigation on the Gotan Limestone and Pondlo Dolomite of the Bilara Group, Marwar Supergroup to decipher the palaeodepositional redox condition. A multivariate- statistical analysis of geochemical data set (δ13C-carb, δ18O-carb, δ13C-org, TOC, V, Ni, Cu, Zn, Mo, Pb, and U) reveals that limestone and dolomite units of the Bilara Group were deposited under two different redox regimes. Dolomite has an average δ13C-org values ~ − 30.0 ‰ and shows a statistically significant negative correlation with 13δ C-carb which indicates an oxygen-depleted closed palaeodepositional environment. Dolomite δ13C-carb also shows a statistically significant nega- tive correlation with redox-sensitive trace elements V, Ce, Pb, and U which infer higher secondary productivity during the dolomite deposition. The limestone has an average δ13C-org values ~ − 28.9 ‰ and δ13C-carb shows a significant positive correlation with δ18O-carb and Sr, whereas δ13C-carb shows a significant negative correlation with V and U. These statistical relationships among the geochemical parameter are indicative of suboxic-to-oxic water column with a relatively higher depth that received a significant input of water discharge from the terrestrial region. This discharge may have brought nutrients and other weathering-derived ions into the basin. -
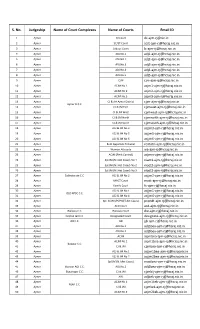
E-Mail Ids of Rajasthan Judiciary
S. No. Judgeship Name of Court Complexes Name of Courts Email ID 1 Ajmer DJ Court [email protected] 2 Ajmer SC/ST Court [email protected] 3 Ajmer Labour Court [email protected] 4 Ajmer ADJ No.1 [email protected] 5 Ajmer ADJ NO.2 [email protected] 6 Ajmer ADJ No.3 [email protected] 7 Ajmer ADJ No.4 [email protected] 8 Ajmer ADJ No.5 [email protected] 9 Ajmer CJM [email protected] 10 Ajmer ACJM No.1 [email protected] 11 Ajmer ACJM No.2 [email protected] 12 Ajmer ACJM No.3 [email protected] 13 Ajmer CJ & JM Ajmer District [email protected] Ajmer D.C.C. 14 Ajmer CJ & JM East [email protected] 15 Ajmer CJ & JM West [email protected] 16 Ajmer CJ & JM North [email protected] 17 Ajmer CJ & JM South [email protected] 18 Ajmer ACJ & JM No.3 [email protected] 19 Ajmer ACJ & JM No.5 [email protected] 20 Ajmer ACJ & JM No.6 [email protected] 21 Ajmer Rent Appellate Tribunal [email protected] 22 Ajmer Women Attrocity [email protected] 23 Ajmer ACJM (Rent Control) [email protected] 24 Ajmer Spl.JM (N.I.Act Cases) No.1 [email protected] 25 Ajmer Spl.JM (N.I.Act Cases) No.2 [email protected] 26 Ajmer Spl.JM (N.I.Act Cases) No.3 [email protected] 27 Ajmer Collectorate C.C. -

Jktlfkku Yksd Lsok Vk;Ksx }Kjk Iz/ Kkuk/;Kid&Ekfo Izf R
jktLFkku yksd lsok vk;ksx }kjk iz/kkuk/;kid&ekfo izfr;ksxh ijh{kk &2018 esa p;fur vk'kkfFkZ;ksa ds inLFkkiu gsrq la'kksf/kr dkmlfyax ofj;rk dkmlfyax esfjV fo'ks"k tkWap jksy ua0 vkosfnr oxZ p;u oxZ uke tUefrfFk irk dkmlfyax fnol ofj;rk dzekWad uEcj oxZ ny dz- 1 134840 366 BC,WE,LD,RG GENF MANJU 1 20-07-1987 WARD NO 01, MANAKSAR, SURATGARH, MANAKSAR, SRI GANGANAGAR, 1 14-10-2019 Rajasthan, 335804 2 190024 428 BC,LD,RG OBCM MOHAN LAL KALBI 1 07-05-1986 KALBIVAS NAVIN BHAWARI POST BHAWARI TEHSIL PINDWARA, BHAWARI, 2 14-10-2019 SIROHI, Rajasthan, 307023 3 168049 531 MB,LD,RG MBCM RAJESH KUMAR GURJAR 1 01-08-1981 DHANI MANDHA, VPO BHAGEGA, NEEM KA THANA, SIKAR, Rajasthan, 3 14-10-2019 332714 4 133150 578 BC,LD,RG OBCM AMANDEEP 1 08-06-1986 VPO KISHANPURA UTTRADHA, TEH SANGARIA, DIST HANUMANGARH, 4 14-10-2019 KISHANPURA UTTRADHA, HANUMANGARH, Rajasthan, 335062 5 180021 629 BC,LD,RG OBCM KESA RAM BISHNOI 1 08-07-1988 VILLAGE MALIYO KA GOLIYA, POST BHADRUNA, TEH SANCHORE, MALIYO KA 5 14-10-2019 GOLIYA, JALORE, Rajasthan, 343041 6 130758 631 BC,LD,RG OBCM VIKRAM SWAMI 1 15-09-1981 VPO.KARWASAR, Karwasar, CHURU, Rajasthan, 331001 6 14-10-2019 7 147570 679 GE,LD,RG GENM SHANKER LAL SHARMA 1 01-05-1979 MUKAM POST-JAISINGHPURA GUARDY, VIA- KALADERA, TEH.-CHOMU, 7 14-10-2019 RAMDEV BABA KI DHANI, JAIPUR, Rajasthan, 303801 8 105779 689 GE,LD,RG GENM HEMENDRA SINGH RATHORE 1 14-11-1976 NEAR TELEPHONE EXCHANGE, GANDHI NAGAR COLONY, GULABPURA, 8 14-10-2019 GULABPURA, BHILWARA, Rajasthan, 311021 9 142328 760 ST,LD,RG STM RAM RATAN MEENA 1 02-10-1982 VPO-KHORA LADKHANI, VIA-GATHWARI, TEH-SHAHPURA, KHORA 9 14-10-2019 LADKHANI, JAIPUR, Rajasthan, 303120 10 132592 763 BC,WE,LD,RG OBCF MANISH 1 20-04-1986 d/o subhash chander yadav, 1 BHD,Hanumangarh,Rajasthan, 1 BHD bhojasar 10 14-10-2019 bhadra, HANUMANGARH, Rajasthan, 335501 11 180542 792 GE,LD,RG GENM HARVINDER SINGH 1 28-10-1980 PLOT NO.- 171, HANWANT - B,B.J.S. -

List of Lime Kiln Units Allowed to Use Pet Coke in Rajasthan (From 01St February to 28 February 2019)
List of Lime Kiln Units allowed to use Pet Coke in Rajasthan (From 01st February to 28 February 2019) Petcoke Opening Petcoke Closing Source of Purchase of Petcoke (Company Purchased Stock of Total in Consumed Stock of S.No. Name and Address of Units (October) Plant Name Names) During the Petcoke in tonnes During the Petcoke in Month in tonnes) Month tonnes) tonnes tonnes) RELIANCE ESSAR IOCL M/s Calcium Lime Industries, Bakaliawas, 1 Lime Kiln 157.704 154.4 106.08 655.42 915.9 1073.6 933 140.604 Gotan Naguar, M/s Lime Light Corporation, Vill.- Bayad, Tehsil 2 Lime Kiln 110.483 66.06 104.98 94.28 265.32 375.803 276 99.803 Merta City ,Naguar, M/s Durga Lime Industries , Vill.- Gotan , 3 Lime Kiln 497.58 181.21 66.74 118.95 366.9 864.48 361.355 503.125 Naguar. M/s Maruti Minerals, Shiv Road , Gotan 4 Lime Kiln 25.14 201.65 0 0 201.65 226.79 136.44 90.35 Naguar, M/s Quality Chemical $ Allied Product, 5 Lime Kiln 236.11 28.48 28.64 120.13 177.25 413.36 116 297.36 Talanpur Road, Gotan-Naguar. M/s Golden Cement Pvt. Ltd. , Vill.- Jawasia, 6 Lime Kiln 0 0 0 0 0 0 0 0 Pipar City , Jodhpur, M/s Sumedha Chemical Industries, Khasra No. 7 Lime Kiln 245.009 93.62 186.01 130.89 410.52 655.529 409.08 246.449 965/1809, Vill.-Dujasar, Khimsar,Naguar. M/s Aditya Chemicals (Unit-I), Pipar City, 8 Lime Kiln 104.16 29.43 25.68 0 55.11 159.27 87.1 72.17 Jodhpur, M/s Aditya Chemicals (Unit-II), Pipar City, 9 Lime Kiln 0 0 0 0 0 0 0 0 Jodhpur, M/s Sumedha Chemicals, Vill.- Dhanapa, Disst. -

Bhadla (Rajasthan)
Draft Initial Environmental Assessment Report Document Stage: Draft Project Number: 49214-002 (IND) December 2016 IND: Solar Transmission Sector Project (Transmission System associated with Solar Power Parks at Bhadla, Rajasthan) Prepared by Power Grid Corporation of India Ltd. for the Asian Development Bank This Draft Initial Environmental Assessment Report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. TABLE OF CONTENTS S.No Title Page ABBREVIATIONS IV WEIGHTS AND MEASURES V EXECUTIVE SUMMARY 1 1.0 INTRODUCTION 4 1.1 Background 4 1.2 The Project 5 2.0 POLICY, LEGAL AND ADMINISTRATIVE FRAMEWORK 7 2.1 National Environmental Requirements 7 2.2 POWERGRID’s ESPP 9 2.3 Asian Development Bank’s Environmental Requirements 10 3.0 DESCRIPTION OF THE PROJECT 11 3.1 Project Justification 11 3.2 Objective and Benefits of The Project 11 3.3 Project Highlights 12 3.4 Scope of Work 13 3.5 Location 13 4.0 DESCRIPION OF EXISTING ENVIRONMENT 16 4.1 Rajasthan State 16 5.0 ANTICIPATED ENVIRONMENTAL IMPACTS AND MITIGATION MEASURES 25 5.1 Project’s Area of Influence 25 5.2 Impacts and Mitigation Measures Due to Location and Design 25