Microbial Dynamic and Growth Potential of Selected Pathogens in Ethiopian Traditional Fermented Beverages Reda Nemo1,2 and Ketema Bacha1*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Partners' Guide to Ethiopia
PARTNERS’ GUIDE TO ETHIOPIA Africa Avenue, one of the main streets in Addis Ababa. One of the main streets in Addis Ababa. Welcome Statement from Dennis Weller Mission Director, USAID Ethiopia Mission Director Dennis Weller USAID/Ethiopia Dear Reader, If you’ve opened this guide, you have already As the home of the African Union and the United demonstrated an interest in development Nations Economic Commission for Africa, Addis opportunities in Ethiopia. That is an important Abeba is already the diplomatic hub of Africa. first step. Assuming Ethiopia continues on its current development track, it has the potential to become Boasting one of the highest GDP growth rates an economic hub. in the world over the past decade, Ethiopia is certainly on the rise. The Government of Ethiopia All of us at the United States Agency for was instrumental in leading that growth and International Development in Ethiopia (USAID) now has ambitious plans to move Africa’s second would like to encourage potential investors to most populous country and one of its poorest to look at the opportunities which Ethiopia offers middle-income status by 2025. In support of that and to consider investing if it makes sense. As a goal, it is investing heavily in its infrastructure and development agency, we like to say that our goal social services. is to one day work ourselves out of a job. That day may still be some way off in Ethiopia, but we’re For our part, USAID’s development portfolio optimistic that the growing involvement of the consists of a wide range of activities designed private sector in Ethiopia’s development will help to create opportunities for Ethiopian citizens. -

Health Benefits of Fermented Foods and Beverages
Food & Culinary Science TAMANG Health Benefits of Fermented Foods and Beverages Health Benefits Health Benets of Fermented Foods and Beverages discusses the functionality and myriad health benets of fermented foods and beverages of the world. It examines health-promoting and therapeutic properties, covering the molecular process of fermentation and the resulting benet to nutritional value and long-term health. Exploring a range of fermented food Health Benefits products from yogurt to tempeh to wine, the book details probiotic activity, degradation of anti-nutritive compounds, and the conversion of substrates into consumable products with enhanced avor and aroma. The diversity of functional microorganisms in fermented foods and beverages of of consists of bacteria, yeasts, and fungi. The most remarkable aspect is the Fermented Foods biological functions and the enhanced health benets due to functional Fermented Foods microorganisms associated with them. Written by a host of international experts, the book highlights the microorganisms in fermented foods and beverages of the world. It collates information based on research articles and and review papers investigating the different health-promoting benets Beverages such as antioxidant functions, allergic reactions suppression, and overall digestion improvement. Possible health benets of fermented foods and beverages include preven- E D I T E D B Y tion of cardiovascular disease, cancer, hepatic disease, gastrointestinal disorders and inammatory bowel disease, hypertension, thrombosis, osteoporosis, allergic reactions, and diabetes. In addition, increasing the JYOTI PRAKASH TAMANG synthesis of nutrient, reducing obesity, increasing immunity, and alleviating lactose intolerance as well as anti-aging and therapeutic values/medicinal and values are among health-related effects attributed to fermented foods. -

The Sacred City of the Ethiopians, Being a Record of Travel and Research in Abyssinia in 1893
The sacred city of the Ethiopians, being a record of travel and research in Abyssinia in 1893 http://www.aluka.org/action/showMetadata?doi=10.5555/AL.CH.DOCUMENT.sip100052 Use of the Aluka digital library is subject to Aluka’s Terms and Conditions, available at http://www.aluka.org/page/about/termsConditions.jsp. By using Aluka, you agree that you have read and will abide by the Terms and Conditions. Among other things, the Terms and Conditions provide that the content in the Aluka digital library is only for personal, non-commercial use by authorized users of Aluka in connection with research, scholarship, and education. The content in the Aluka digital library is subject to copyright, with the exception of certain governmental works and very old materials that may be in the public domain under applicable law. Permission must be sought from Aluka and/or the applicable copyright holder in connection with any duplication or distribution of these materials where required by applicable law. Aluka is a not-for-profit initiative dedicated to creating and preserving a digital archive of materials about and from the developing world. For more information about Aluka, please see http://www.aluka.org The sacred city of the Ethiopians, being a record of travel and research in Abyssinia in 1893 Author/Creator Bent, J. Theodore Date 1896 Resource type Books Language English Subject Coverage (spatial) Horn of Africa, Ethiopia, Axum, Eritrea Source Smithsonian Institution Libraries, DT379 .B47 1896X/916.3 B475s Description Contents. I: Arrival in Ethiopia. II: Stay at Asmara. III: Expedition to the monastery of Bizen. -
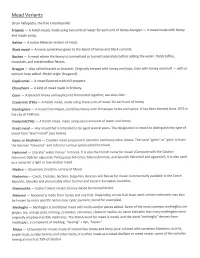
Mead Variants
Mead Variants (From Wikipedia, the free encyclopedia) Tr6jniak - A Polish mead, made using two units of water for each unit of honey Acerglyn - A mead made with honey and maple syrup. Balche - A native Mexican version of mead. Black mead - A name sometimes given to the blend of honey and black currants. Bochet - A mead where the honey is caramelized or burned separately before adding the water. Yields toffee, chocolate, and marshmallow flavors. Braggot - Also called bracket or brackett. Originally brewed with honey and hops, later with honey and malt - with or without hops added. Welsh origin (bragawd). Capsicumel - A mead flavored with chili peppers. Chouchenn - A kind of mead made in Brittany. Cyser - A blend of honey and apple juice fermented together; see also cider. Czw5rniak (TSG) - A Polish mead, made using three units of water for each unit of honey Dandaghare - A mead from Nepal, combines honey with Himalayan herbs and spices. lt has been brewed since 1972 in the city of Pokhara. Dw6jniak(Tsc) - A Polish mead, made using equal amounts of water and honey Great mead - Any mead that is intended to be aged several years. The designation is meant to distinguish this type of mead from "short mead" (see below). Gverc or Medovina - Croatian mead prepared in Samobor and many other places. The word "gverc" or "gvirc' is from the German "Gewiirze" and refers to various spices added to mead. Hydromet - Literally "water-honey" in Greek. lt is also the French name for mead. (Compare with the Catalan hidromel, Galician aiguamel, Portuguese hidromel, ltalian idromele, and Spanish hidromiel and aguamiel). -

Common Mead Variants Acerglyn: a Mead Made with Honey Melomel: Melomel Is Made from Short Mead: Also Called “Quick and Maple Syrup
BEVERAGE CLASS mead beverage class Common Mead Variants Acerglyn: A mead made with honey Melomel: Melomel is made from Short mead: Also called “quick and maple syrup. honey and any fruit. Depending on the mead.” A type of mead recipe that is fruit base used, certain melomels may meant to age quickly, for immediate Black mead: A name sometimes also be known by more specific names consumption. Because of the techniques given to the blend of honey and (see cyser and pyment for examples). used in its creation, short mead shares blackcurrants. some qualities found in cider (or Metheglin: Metheglin is Bochet: even light ale)—primarily that it is A mead where the honey traditional mead with herbs or spices is caramelized or burned separately effervescent, and often has a cidery taste. added. Some of the most common It can also be champagne-like. before adding the water. Yields metheglins are ginger, tea, orange peel, toffee, caramel, chocolate and toasted nutmeg, coriander, cinnamon, cloves Show mead: A term which has marshmallow flavors. or vanilla. Its name indicates that many come to mean “plain” mead, that which Braggot: Originally brewed with metheglins were originally employed as has honey and water as a base, with no honey and hops, later with honey and folk medicines. fruits, spices or extra flavorings. malt—with or without hops added. Pyment: Pyment blends honey and Tej: Tej is an Ethiopian and Eritrean Capsicumel/Capsumel: A red or white grapes and/or grape juice. mead, fermented with wild yeasts and the addition of gesho. mead flavored with chile peppers (the Rhodomel: Rhodomel is made peppers may be hot or mild). -

View in 1986: "The Saccharine Sweet, Icky Drink? Yes, Well
Yashwantrao Chavan Maharashtra Open University V101:B. Sc. (Hospitality and Tourism Studies) V102: B.Sc. (Hospitality Studies & Catering Ser- vices) HTS 202: Food and Beverage Service Foundation - II YASHWANTRAO CHAVAN MAHARASHTRA OPEN UNIVERSITY (43 &Øا "••≤°• 3•≤©£• & §°© )) V101: B. Sc. Hospitality and Tourism Studies (2016 Pattern) V102: B. Sc. Hospitality Studies and Catering Services (2016 Pattern) Developed by Dr Rajendra Vadnere, Director, School of Continuing Education, YCMOU UNIT 1 Non Alcoholic Beverages & Mocktails…………...9 UNIT 2 Coffee Shop & Breakfast Service ………………69 UNIT 3 Food and Beverage Services in Restaurants…..140 UNIT 4 Room Service/ In Room Dinning........................210 HTS202: Food & Beverage Service Foundation -II (Theory: 4 Credits; Total Hours =60, Practical: 2 Credits, Total Hours =60) Unit – 1 Non Alcoholic Beverages & Mocktails: Introduction, Types (Tea, Coffee, Juices, Aerated Beverages, Shakes) Descriptions with detailed inputs, their origin, varieties, popular brands, presentation and service tools and techniques. Mocktails – Introduction, Types, Brief Descriptions, Preparation and Service Techniques Unit – 2 Coffee Shop & Breakfast Service: Introduction, Coffee Shop, Layout, Structure, Breakfast: Concept, Types & classification, Breakfast services in Hotels, Preparation for Breakfast Services, Mise- en-place and Mise-en-scene, arrangement and setting up of tables/ trays, Functions performed while on Breakfast service, Method and procedure of taking a guest order, emerging trends in Breakfast -

Free Download, Last Accessed October 30, 2013
business producing high-value foods business producing Setting up and running Setting up and running a small-scale business producing a small-scale high-value foods Opportunities in food processing Opportunities in food processing a series Opportunities in Food Processing A handbook for setting up and running a small-scale business producing high-value foods Contributing authors: Yeshiwas Ademe, Barrie Axtell, Peter Fellows, Linus Gedi, David Harcourt, Cécile La Grenade, Michael Lubowa and Joseph Hounhouigan Edited by: Peter Fellows and Barrie Axtell Midway Associates Published by CTA (2014) About CTA The Technical Centre for Agricultural and Rural Cooperation (CTA) is a joint international institution of the African, Caribbean and Pacific (ACP) Group of States and the European Union (EU). Its mission is to advance food and nutritional security, increase prosperity and encourage sound natural resource management in ACP countries. It provides access to information and knowledge, facilitates policy dialogue and strengthens the capacity of agricultural and rural development institutions and communities. CTA operates under the framework of the Cotonou Agreement and is funded by the EU. For more information on CTA, visit www.cta.int or contact: CTA PO Box 380 6700 AJ Wageningen The Netherlands E-mail: [email protected] Citation: Fellows, P.J. and Axtell, B. (Eds), 2014. Opportunities in Food Processing: A handbook for setting up and running a small- scale business producing high-value foods. Wageningen: ACP-EU Technical Centre for Agricultural and Rural Cooperation (CTA). ISBN 978-92-9081-556-3 Copyright © 2014 CTA, Wageningen, The Netherlands. All rights reserved. No part of this publication may be reproduced, stored in retrieval systems or transmitted in any form or by any means without prior permission of CTA. -

ECFG-Ethiopia-2020R.Pdf
About this Guide This guide is designed to prepare you to deploy to culturally complex environments and achieve mission objectives. The fundamental information contained within will help you understand the cultural dimension of your assigned location and gain skills necessary for success. (Photo a courtesy of Wikimedia). The guide consists of 2 parts: ECFG Part 1 introduces “Culture General,” the foundational knowledge you need to operate effectively in any global environment. Part 2 presents “Culture Specific” Ethiopia, focusing on Ethiopia unique cultural features of Ethiopian society and is designed to complement other pre-deployment training. It applies culture-general concepts to help increase your knowledge of your assigned deployment location. For further information, visit the Air Force Culture and Language Center (AFCLC) website at www.airuniversity.af.edu/AFCLC/ or contact AFCLC’s Region Team at [email protected]. Disclaimer: All text is the property of the AFCLC and may not be modified by a change in title, content, or labeling. It may be reproduced in its current format with the expressed permission of the AFCLC. All photography is provided as a courtesy of the US government, Wikimedia, and other sources as indicated. GENERAL CULTURE CULTURE PART 1 – CULTURE GENERAL What is Culture? Fundamental to all aspects of human existence, culture shapes the way humans view life and functions as a tool we use to adapt to our social and physical environments. A culture is the sum of all of the beliefs, values, behaviors, and symbols that have meaning for a society. All human beings have culture, and individuals within a culture share a general set of beliefs and values. -

Mead 101 W Recipes
MEAD 101 Mead: at least 50% of fermentables from honey Strength: Hydromel (<1.080 OG), Standard (1.080 – 1.120), Sack (>1.120) Effervescence: Still, Petulent, Sparkling Sweetness: Dry (<1.010 FG), Semi-sweet (1.010 – 1.025), Sweet (>1.025) Types of Mead Traditional: Honey, Water & Yeast Varietals include orange blossom, clover, sourwood, tupelo and many others. Melomel: Fruit meads, several are popular enough to have special names. Cyser: apple/cider Pyment: grapes/juice Metheglin: Spice meads Mulling spices: Allspice, cinnamon, cloves, ginger, nutmeg Beer spices: Coriander, cardamom, curacao orange, woodruff, etc. Mellow spices: Vanilla, chocolate, anise, sassafras, maple syrup Italian spices: Oregano, basil, thyme, bay, sage, rosemary, garlic Flower Petal Metheglin: Rose petals (Rhodimel), dandelion, lavendar, tea blends, heather tips, hop cones (Miomel), honeysuckle, elderberry flowers. Peppery: White/black pepper, mint, lemon grass, curry powder, grains of paradise, juniper berries, spruce, mustard seed, fennel, tumeric, cumin. Chile mead (capsicumel) varies in heat. Braggot: malt/extract (hops not required) Yeasts Lalvin K1V-1116: light & fruity meads Wyeast 4184: sweet meads “ DV-10: high gravity meads “ 4632: dry meads “ EC-1118: high gravity, neutral flavor WLP720: sweet meads Red Star Premiere Cuvee: dry meads Lalvin D-47: Med. to sweet meads, earthy tones Red Star Montrachet: dry meads “ 71B: light & fruity, med-dry meads, fast Glossary Metheglin: Mead with spices or extracts Acerglin: Mead with maple syrup Miodomel: Mead with -

Aksum an African Civilisation of Late Antiquity
Aksum An African Civilisation of Late Antiquity Stuart Munro-Hay Dedicated to the late H. Neville Chittick Aksum: An African Civilisation of Late Antiquity was first published in 1991. Some errors have been corrected in this edition. © Stuart Munro-Hay 1991 [put online with permission by Alan Light, <[email protected]>] [A number of readers have wanted to contact Mr. Munro-Hay. His current address is at aol.com, user name is munrohay. I'm using that format to try to keep him from getting spam.] British Library Cataloguing in Publication Data Munro-Hay, S. C. (Stuart C), 1947- Aksum: an African civilization of late antiquity. 1. Axumite Kingdom, history I. Title 963.4 Contents Chronological Chart Preface 1. Introduction 2. Legend, Literature, and Archaeological Discovery 1. The Legends of Aksum 2. Aksum in Ancient Sources 3. The Rediscovery of Aksum in Modern Times 3. The City and the State 1. The Landscape 2. Origins and Expansion of the Kingdom 3. The Development of Aksum; an Interpretation 4. Cities, Towns and Villages 5. The Inhabitants 6. Foreign Relations 4. Aksumite History 1. The Pre-Aksumite Period 2. Comparative Chronological Chart; Rulers, Sources and Sites 3. Period 1; Early Aksum until the Reign of Gadarat 4. Period 2; Gadarat to Endubis 5. Period 3; Endubis to Ezana 6. Period 4; Ezana after his Conversion, to Kaleb 7. Period 5; Kaleb to the End of the Coinage 8. The Post-Aksumite Period 5. The Capital City 1. The Site 2. The Town Plan 3. Portuguese Records of Aksum 4. Aksumite Domestic Architecture 5. -

Isolation and Screening of Antibacterial Producing Lactic Acid Bacteria from Traditionally Fermented Drinks (“Ergo” and “Tej”) in Gondar Town, Northwest Ethiopia
Global Research Journal of Public Health and Epidemiology: ISSN-2360-7920, Vol. 1(3): pp 018-022, March, 2014. Copyright © 2014 Spring Journals Full Length Research Paper Isolation and screening of antibacterial producing lactic acid bacteria from traditionally fermented drinks (“Ergo” and “Tej”) in Gondar town, Northwest Ethiopia Lelise A, Belaynesh G, Mulubrhan M, Kedija S, Endashaw B and *Abebe B Department of Biology, Faculty of Natural and Computational Sciences, University of Gondar, Gondar, Ethiopia *Corresponding Author’s E-mail: [email protected] Accepted 28th February, 2014 Traditional fermented beverages that contain lactic acid bacteria have antimicrobial activity for various human pathogens and used for preservation of beverages for a long time. However, there is no scientific report on the antimicrobial activity of lactic acid bacteria isolated from different beverages in Gondar town. The objective of the study was to isolate and screen lactic acid bacteria that show antibacterial activity against clinical and standard human pathogens. Samples of “Ergo” and “Tej” were collected from Arada, Azezo and Kebele 18. A volume of 0.1ml suspension from 10-6 dilution of each sample was spread on MRS agar plates and incubated both in aerobic and anaerobic conditions. Morphologically distinct colonies were subcultured to MRS broth and incubated at 37 0C for 72 hrs in thermostat water bath. The broth culture was centrifuged and the supernatant was used to check the antimicrobial activity against clinical and standard pathogens using agar well diffusion assay. The amount of lactic acid and H2O2 produced by the isolates during fermentation was determined using color change after titration. -

Sweet Treats Around the World This Page Intentionally Left Blank
www.ebook777.com Sweet Treats around the World This page intentionally left blank www.ebook777.com Sweet Treats around the World An Encyclopedia of Food and Culture Timothy G. Roufs and Kathleen Smyth Roufs Copyright 2014 by ABC-CLIO, LLC All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except for the inclusion of brief quotations in a review, without prior permission in writing from the publisher. The publisher has done its best to make sure the instructions and/or recipes in this book are correct. However, users should apply judgment and experience when preparing recipes, especially parents and teachers working with young people. The publisher accepts no responsibility for the outcome of any recipe included in this volume and assumes no liability for, and is released by readers from, any injury or damage resulting from the strict adherence to, or deviation from, the directions and/or recipes herein. The publisher is not responsible for any readerÊs specific health or allergy needs that may require medical supervision or for any adverse reactions to the recipes contained in this book. All yields are approximations. Library of Congress Cataloging-in-Publication Data Roufs, Timothy G. Sweet treats around the world : an encyclopedia of food and culture / Timothy G. Roufs and Kathleen Smyth Roufs. pages cm Includes bibliographical references and index. ISBN 978-1-61069-220-5 (hard copy : alk. paper) · ISBN 978-1-61069-221-2 (ebook) 1. Food·Encyclopedias.