Academic Journal of Chemistry Colorimetric Determination Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Diacetate
Technical Data Sheet Ref.: 2019_12v09 Sodium Diacetate Stability Description SodiumDiacetate is stable for 3 years from Sodium Diacetate is available as a free date of production. Physical stability and flowing white crystal, containing appearance may change before the end of approximately equimolar amounts of shelf-life if not stored single-stacked in Sodium Acetate and acetic acid. closed original packaging, dry and at room temperature. PRODUCT PROPERTIES Handling Product is classified as irritant. Always Product name Sodium Diacetate check the Safety Data Sheet and label Formula CH3COONaCH3COOH before using the product. Molecular weight 142.08 g/mol CAS No. 126-96-5 Packaging EINECS No. 2048149 Sodium Diacetate crystal is packed in 900 kg big bags. HS code US 2915.29.5000 HS code EU 2915.29.00 Safety precautions Please see the Safety Data Sheet before handling the material. Product grades Sodium Diacetate has a free acetic acid content of 40% - 43%. Application Sodium Diacetate is used in haemodialysis. The product is also used in other applications where it is advantageous to use a solid source of free acetic acid, e.g. for safer handling and reduced transportation costs. Warranty. This information herein is offered as a guide and is believed to be accurate and reliable as of the date of the printing. The values given are not to be considered as a warranty and they are subject to change without prior notice. For additional information regarding our products or for information concerning current specifications, please contact our Technical Service. Niacet Corporation www.niacet.com Niacet b.v. 400, 47th Street P.O. -

Effects of Sodium Citrate Plus Sodium Diacetate and Buffered Vinegar on Escherichia Coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef
359 Journal of Food Protection, Vol. 74, No. 3, 2011, Pages 359–364 doi:10.4315/0362-028X.JFP-10-294 Copyright G, International Association for Food Protection Effects of Sodium Citrate plus Sodium Diacetate and Buffered Vinegar on Escherichia coli O157:H7 and Psychrotrophic Bacteria in Brine-Injected Beef AMUDHAN PONRAJAN,1,2 MARK A. HARRISON,1 JACOB R. SEGERS,2 BRADLEY K. LOWE,2 RUSSELL O. MCKEITH,2 T. DEAN PRINGLE,2 KARINA G. MARTINO,1 JAKE H. MULLIGAN,1 AND ALEXANDER M. STELZLENI2* Downloaded from http://meridian.allenpress.com/jfp/article-pdf/74/3/359/1685103/0362-028x_jfp-10-294.pdf by guest on 01 October 2021 1Department of Food Science and Technology and 2Department of Animal and Dairy Sciences, University of Georgia, Athens, Georgia 30602, USA MS 10-294: Received 15 July 2010/Accepted 4 December 2010 ABSTRACT The objective of this research was to examine the effects of sodium citrate plus sodium diacetate or buffered vinegar on Escherichia coli O157:H7 and psychrotrophic bacteria when incorporated in brine solutions for injected beef. Two experiments were conducted in which 30 top rounds and 30 top sirloins were injected (110%) to contain (i) 0.5% sodium chloride and 0.4% sodium tripolyphosphate as the control (CNT); (ii) CNT with a 1% solution of 80% sodium citrate plus 20% sodium diacetate (SCzD); or (iii) CNT with 2% buffered vinegar (VIN) in the final product. For the E. coli challenge, muscles were surface inoculated to target 6 log CFU/cm2. After injection and 10 days of storage in a vacuum package (4uC), one half of each muscle was sampled raw and the other half was cooked to an internal temperature of 60uC with a 12-min hold. -

Shale Gas and Groundwater Quality
Shale Gas and Groundwater Quality A literature review on fate and effects of added chemicals Alette Langenhoff 1202141-008 © Deltares, 2011 1202141-008-ZWS-0001, 28 December 2011, final Contents 1 Introduction 1 2 The process of fracturing or fracking 5 3 The use of chemicals 7 4 Polyacrylamide 8 4.1 Aerobic degradation of polyacrylamide 8 4.2 Anaerobic degradation 10 4.3 Chemical or physical removal 10 4.4 Conclusion on removal of polyacrylamide 10 5 Glutaraldehyde 12 5.1 Biocide 12 5.2 Biodegradation 12 5.3 Chemical inactivation of glutaraldehyde 13 6 Conclusions 14 7 References 15 Appendices 17 Appendices A Chemicals identified in hydraulic fracturing fluid and flowback/produced water (EPA, 2011). A-1 B Fracturing fluid ingredients and common uses (Europe Unconventional Gas 2011) B-1 C Properties of Polyacrylamide (source: Wikipedia) C-1 D Properties of Glutaraldehyde (source: Wikipedia) D-1 Shale Gas and Groundwater Quality i 1202141-008-ZWS-0001, 28 December 2011, final 1 Introduction Shale gas is a so-called unconventional sources of natural gas, and is one of the most rapidly expanding trends in onshore domestic oil and gas exploration and production today (Fig. 1 and 2). Shale gas is present in hydrocarbon rich shale formations. Shallow gas is commonly defined as gas occurrences in unconsolidated sediments of Tertiary age (often down to depths of 1000 m below surface). The occurrences are positively associated with thick Neogene sediments and are often trapped in anticlinal structures associated with rising salt domes (Muntendam-Bos et al, 2009). Shale has low matrix permeability, so gas production in commercial quantities requires fractures to provide permeability. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Listeria Monocytogenes on Frankfurters Treated with Organic Acid Salts Zheng Lu Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2004 Inhibition of Listeria monocytogenes on frankfurters treated with organic acid salts Zheng Lu Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agriculture Commons, Animal Sciences Commons, and the Food Science Commons Recommended Citation Lu, Zheng, "Inhibition of Listeria monocytogenes on frankfurters treated with organic acid salts " (2004). Retrospective Theses and Dissertations. 1182. https://lib.dr.iastate.edu/rtd/1182 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. ® UMI Inhibition of Listeria monocytogenes on frankfurters treated with organic acid salts by Zheng Lu A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Meat Science Program of Study Committee: Joseph Sebranek, Major Professor Theodore Bailey James Dickson Aubrey Mendonca David Topel Iowa State University Ames, Iowa 2004 Copyright © Zheng Lu, 2004. All rights reserved. UMI Number: 3158356 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

SAFETY DATA SHEET R Sodium Diacetate and Ethanol Aqueous Solution
Conforms to HCS 2012 - United States and Canada WHMIS 2015 SAFETY DATA SHEET R Sodium Diacetate and Ethanol Aqueous Solution Section 1. Identification GHS product identifier : Sodium Diacetate and Ethanol Aqueous Solution Other means of : Not available. identification Part number : 2606955 Product description : CadenceTM single pass Tangential Flow Filtration Modules made with Delta membranes are stored and shipped liquid wet with an aqueous solution of ethanol and sodium diacetate contained within a sealed plastic bag. This solution is employed with the following Cadence part numbers CD0#TD**** (where # is the molecular cut off in kilo Daltons). Product type : Liquid. Relevant identified uses of the substance or mixture and uses advised against Identified uses : CadenceTM single pass Tangential Flow Filtration Modules made with Delta membranes are stored and shipped liquid wet with an aqueous solution of ethanol and sodium diacetate contained within a sealed plastic bag. This solution is employed with the following Cadence part numbers CD0#TD**** (where # is the molecular cut off in kilo Daltons). Before use an initial flush is required prior to operation to remove the wetting solution. Supplier : Pall Corporation 225 Marcus Boulevard Hauppauge, NY 11788, USA Tel: 1-631-478-6254 Web: www.Pall.com Supplier's details : Pall Corporation 3450 Ridgeway Drive, Unit 6 Mississauga, Ontario L5L 0A2 Tel: 1-905-542-0330 Emergency telephone : CHEMTREC, U.S. : 1-800-424-9300 number (with hours of International: +1-703-527-3887 operation) 24 hours/day, 7 days/week Country of Origin: : Made in USA. Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Safety Data Sheet (Sds) (1272/2008/Ce)
SAFETY DATA SHEET (SDS) (1272/2008/CE) Trade N ame: Edition: May 29, 2015 SODIUM DIACETATE Version: 6/en 1. IDENTIFICATION OF THE SUBSTANCE / PREPARATION AND COMPANY / UNDERTAKING Trade name Sodium Diacetate Synonyms Sodium acid acetate, powdered acetic acid, sodium acetate bound to one mole of acetic acid. Recommended use Use as a preservative in food, feed and pharmaceutical applications. Use according to local and restriction on use regulations. Company Macco Organiques Inc., 100 McArthur, Valleyfield, Qc, Canada, J6S 4M5 Responsable service Tel: (450) 371-1066 Fax: (450) 371-5519 [email protected] http://www.macco.ca Emergency phone CANUTEC CHEMTREC, U.S. CANUTEC numbers (613) 996-6666 (800) 424-9300 (613) 996-6666 2. HAZARDS IDENTIFICATION The substance is classified according to European Union (EU) directives 1272/2008, in replacement for 67/548/EEC including amendments and 1999/45/EC. Classification Cause eye irritation, category 2, Harmful in contact with eye. Cause skin irritation, category 2, Harmful in contact with skin. Labeling type instruction Pictogram Signal word Warning H319 Causes eye irritation. Hazard statement H315 Causes skin irritation. Precautionary Statements Prevention P280: Wear eye protection, protective gloves and clothing. Response P305+ IF IN EYES: Rinse cautiously with water for several minutes. P351+ Remove contact lenses, if present and easy to do. Continue P338 rinsing. P302+ IF ON SKIN: Wash with plenty of water. P362+ Take off contaminated clothing and wash before reuse. P364 P352 Call a doctor if you feel unwell. Other hazards Combustible dust. May form explosive mixture with air. Authorisation Date: 29 MA 15 Page 1 of 10 Prepared by: HL Authorized by: JJRD SAFETY DATA SHEET (SDS) (1272/2008/CE) Trade N ame: Edition: May 29, 2015 SODIUM DIACETATE Version: 6/en 3. -

Chemical Compatibility Storage Group
CHEMICAL SEGREGATION Chemicals are to be segregated into 11 different categories depending on the compatibility of that chemical with other chemicals The Storage Groups are as follows: Group A – Compatible Organic Acids Group B – Compatible Pyrophoric & Water Reactive Materials Group C – Compatible Inorganic Bases Group D – Compatible Organic Acids Group E – Compatible Oxidizers including Peroxides Group F– Compatible Inorganic Acids not including Oxidizers or Combustible Group G – Not Intrinsically Reactive or Flammable or Combustible Group J* – Poison Compressed Gases Group K* – Compatible Explosive or other highly Unstable Material Group L – Non-Reactive Flammable and Combustible, including solvents Group X* – Incompatible with ALL other storage groups The following is a list of chemicals and their compatibility storage codes. This is not a complete list of chemicals, but is provided to give examples of each storage group: Storage Group A 94‐75‐7 2,4‐D (2,4‐Dichlorophenoxyacetic acid) 94‐82‐6 2,4‐DB 609-99-4 3,5-Dinitrosalicylic acid 64‐19‐7 Acetic acid (Flammable liquid @ 102°F avoid alcohols, Amines, ox agents see SDS) 631-61-8 Acetic acid, Ammonium salt (Ammonium acetate) 108-24-7 Acetic anhydride (Flammable liquid @102°F avoid alcohols see SDS) 79‐10‐7 Acrylic acid Peroxide Former 65‐85‐0 Benzoic acid 98‐07‐7 Benzotrichloride 98‐88‐4 Benzoyl chloride 107-92-6 Butyric Acid 115‐28‐6 Chlorendic acid 79‐11‐8 Chloroacetic acid 627‐11‐2 Chloroethyl chloroformate 77‐92‐9 Citric acid 5949-29-1 Citric acid monohydrate 57-00-1 Creatine 20624-25-3 -
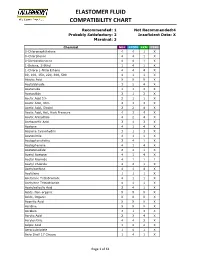
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

“Inert” Ingredients Used in Organic Production
“Inert” Ingredients Used in Organic Production Terry Shistar, PhD A Beyond Pesticides Report he relatively few registered pesticides allowed in organic production are contained in product formulations with so-called “inert” ingredients that are not disclosed on the T product label. The “inerts” make up the powder, liquid, granule, or spreader/sticking agents in pesticide formulations. The “inerts” are typically included in products with natural or synthetic active pesticide ingredients recommended by the National Organic Standards Board (NOSB) and listed by the National Organic Program (NOP) on the National List of Allowed and Prohibited Substances. Any of the pesticides that meet the standards of public health and environmental protection and organic compatibility in the Organic Foods Production Act (OFPA) may contain “inert” ingredients. Because the standards of OFPA are different from those used by the U.S. Environmental Protection Agency (EPA) to regulate pesticides and given changes in how the agency categorizes inerts, the NOSB has adopted a series of recommendations since 2010 that established a substance review process as part of the sunset review. NOP has not followed through on the Board’s recommendations and, as a result, there are numerous materials in use that have not been subject to OFPA criteria. This report (i) traces the history of the legal requirements for review by the NOSB, (ii) identifies the universe of toxic and nontoxic materials that make of the category of “inerts” used in products permitted in organic production, and (iii) suggests a path forward to ensure NOSB compliance with OFPA and uphold the integrity of the USDA organic label. -

HF Chemical Disclosure Reference List
Nevada Division of Minerals Hydraulic Fracture Chemical Disclosure Reference List (in CASRNTM numerical order) Carcinogenic Chemical EPA's Pesticide Agent Classified API Well Avg. Conc. Abstracts InertFinder Listing Chemical Name (* Existing Chemical (Group 1 or 2) by CA Prop.65 Listed NIOSH REL OSHA PEL Usage Count In HF Service Registry Other Uses If Approved for indicates Trade Name or ingredient is Trade Product Function Chemical Purpose on EPA TSCA list the IARC Agent (TWA ppm) (TWA ppm) West of Fluid (% Number (2) Food, NonFood, Secret) (3) Monographs - (6) (7) (7) Long. 104 by mass) (CASRN™) Fragrance Use (V. 1-109) (8) (8) (1) (4) Row # (5) Eliminates bacteria in the water that produces 2-Bromo-2-nitro-1,3-propanediol 000052-51-7 Biocide Preservative in cosmetics and toiletries Yes F, NF No No NL NL 1 corrosive by-products 514 0.00172 2 Glycerine 000056-81-5 Crosslinker Cosmetics, hand lotions, pharmaceuticals Yes F, NF No No NA NA 2,312 0.03336 Paints, detergents, air fresheners, numerous skin, face Propylene glycol 000057-55-6 Yes F, NF, Fr No No NL NL 3 and hair care products; special effects fog 1,960 0.00992 Used in baby formula, skin cleansers, anti-wrinkle Lactose 000063-42-3 Stabilizer Product stabilizer Yes F, NF No No NL NL 4 creams; anti-fungal aquarium treatments 17 0.00764 Alcoholic beverages, pharmaceuticals, perfumes, Ethanol 000064-17-5 Surfactant Product stabilizer and / or winterizing agent. Yes F, NF, Fr No No 1000 1000 5 solvents, dehydration, rubbing alcohol 6,205 0.02972 6 Formic Acid 000064-18-6 Corrosion